When we speak of magnetic polymers we refer, in a broad sense, to functional polymers with magnetic properties, prepared by including magnetic powders such as Fe3O4 or CoFe2O4 in polymeric matrices that often include epoxies, polyurethanes and polyimides; but although attempts have been made to produce polymers that behave like magnets for various applications, they generally have weaker magnetic properties than cast or synthesized magnets.
Scientists develop a magnetically responsive polymer. Source: Image elaborated in powerpoint.
Among the ways to manufacture these magnetic polymers we can mention, by the association of polymers with metal ions and by the incorporation of ferromagnetic particles and metal oxides. Regarding the second way, usually plastic resins are mixed with magnetic metal oxides, so while the polymer is used as a matrix, the magnetic property is provided by the metal oxides included in it.
Regarding the latter, a group of scientists at Flinders University's Chalker Research Laboratory has prepared a new multifunctional material made by hot-pressing a sulfur-rich polymer in the presence of γ-Fe2O3 nanoparticles. The polymer was prepared by direct copolymerization of elemental sulfur and canola vegetable oil, and by pressing, the embedding of magnetic iron particles was achieved.
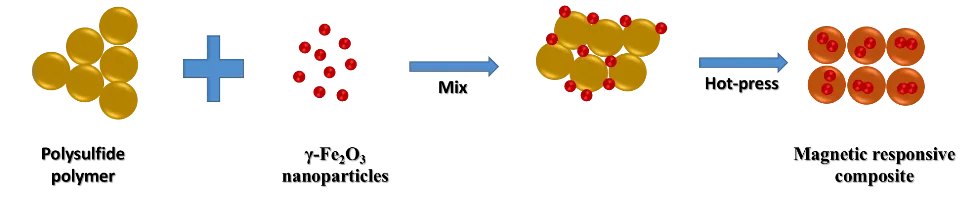
General scheme of the method. Source: Image elaborated in powerpoint.
The iron particles not only give the material magnetic properties, allowing it to be moved at a distance with another magnet, but also give it the ability to be heated quickly by microwaves, which allows for a moldable material that can be cured to obtain the desired solid shape in a matter of seconds.
According to the research team, this versatile material has multiple applications, such as the production of lightweight components for soft electronics, recyclable construction material and even for water purification, it is even recyclable, being able to be crushed to shape it again. The characteristics of this new material were published in the journal Polymer Chemistry.
The material was tested as a mercury sorbent capable of magnetic recovery, for example, it was used to bind mercury present in mining waste and then recover the mercury-bound material with a magnet. Its capacity as a construction material was also measured, when used to make a solenoid valve by molding the material and curing the fabricated component in a microwave oven, this component was not only molded quickly but was also only one-tenth the weight of the metal component.
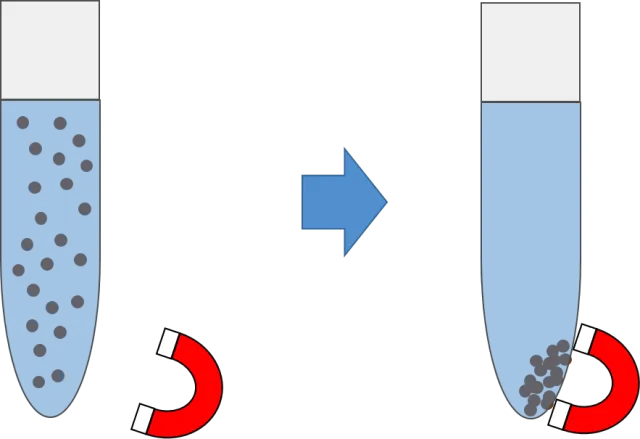
The material was used to remove mercury from a sample of mining waste. Source: Image elaborated in powerpoint.
The study illustrates a growing field for the fabrication of magnetically responsive polymers, which could in the future replace heavy metal magnets used in electronics, or be employed in the growing flexible electronics industry, even opening the frontier for the use of sulfur-rich polymers in different applications, from electronics, lightweight machine components and even environmental remediation.
We can certainly foresee great potential in these polymers with magnetic response, since we use magnets on a daily basis, being part of everyday devices such as phones, headphones and even on the refrigerator door. However, they are hard magnets, which limits their use in various applications where flexibility or a very specific geometric shape is required.
Well friends, I hope you liked the information. See you next time!