Well, this was an unexpected surprise from my department supervisor.
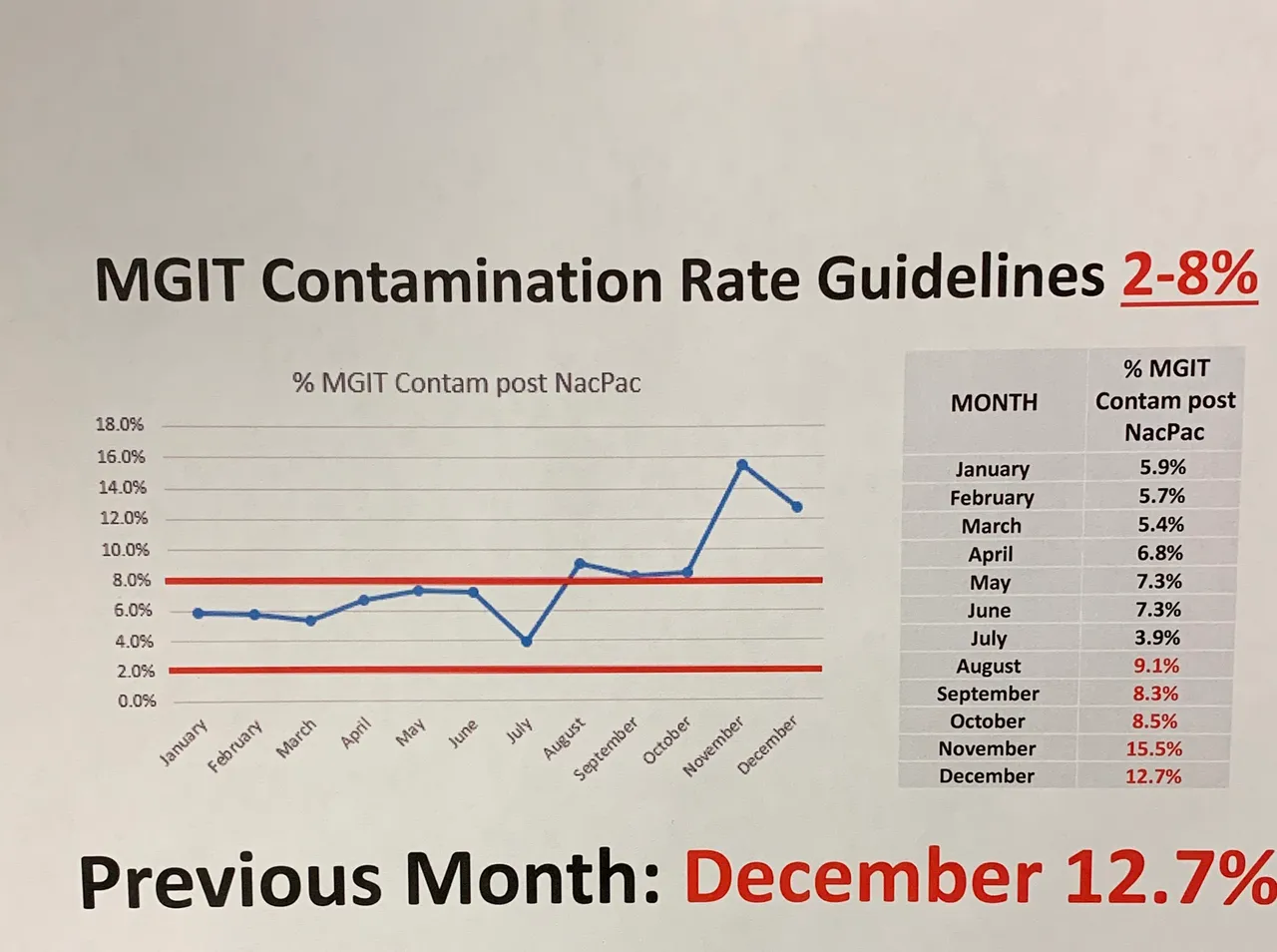
Without knowing anything, one could tell something terrible had happened. Our quality control is outside of accepted range. Internal investigations took place to figure out what went wrong for months.
The percentages you see here relates to a protocol for isolating acid fast bacilli (AFB). This class of bacteria includes those that cause tuberculosis and leprosy. This post won't discuss the workflow in details, but decontamination is a key step in the process. This is due to their slow growing nature compared to other microbes.
The protocol is also used in isolating deadly organisms such as Nocardia species.
The problem
My supervisor isolated the issue. The problem came from the decontamination reagent. At my lab, we use NAC-PAC Red from AlphaTec to process our non-sterile samples.

Another product photo you see.
As you can see from the image, the product includes a vial of pellets next to the bottle. The pellets are vital part of the solution. We have found a surplus of them in our storage. This suggested that some staff members were not preparing the reagents as instructed. This meant that many patient samples winded up having microbes we don't want growing.
What does this mean for the lab?
This means the lab is spending more time decontaminating samples than expected. We were wasting time and resources per test. This increases the number of false positive cultures in our work flow.
I sounded like a corporate shill in that paragraph, but things aren't cheap. And it sucks when you have to spend more resources when you didn't have to.
To remedy the blunder, every staff in the department will receive retraining. In addition, every NAC-PAC bottle will have its corresponding pellets taped to it from now on.
I haven't heard what the medical director has to say in this matter. I know my supervisor has increased the frequency of reporting those statistics. He is definitely going to compile those specific stats more than once a year going forward.
What does this mean for the patients?
Having to do extra decontamination steps affects turn around time. This means we cannot eliminate the possibility of AFBs as soon as we'd like. While cultures for AFB can last up to six weeks, this folly increases the average turn around time.
On the grand scheme of things, we are within the accepted time frame. But, this means the patients would have to wait longer before receiving treatments. In health care, the philosophy is to reduce the patients' suffering, not prolonging it.
The most important thing is, the mistake did not cost the accuracy of our results. It was definitely annoying to do more work than needed.
What does this mean for physicians?
They may not know any better. In my previous posts, I have mentioned that clinicians don't often know the lab processes. But, I would imagine it is frustrating for doctors to wait for results on an ill patient.
The only physician I'm worried about is our medical director. It's gonna be fun hearing from him. We have an early scheduled department meeting coming up.
Bonus
On the chart, you may have noticed that the accepted range is 2-8%. You might be wondering what happens when you go below that mark? The short answer is that it's also bad because that means we have false negative cultures. False negatives mean we over-decontaminated patient samples and killed all the microbes.
In my limited understanding, false negatives are much worse in this scenario. A lot of the AFB infections have long-term consequences if left alone. I want to mention that I don't know what precautions clinicians follow to mitigate that sort of mistake on the lab's end.