As we well know, the growing increase in energy demand and the environmental problems derived from the use of fossil fuels have driven the exploration of different renewable energy sources, whose purpose is to mitigate the environmental impact generated by traditional energy sources, such as oil and gas.
In this sense, many studies have focused on the use of residual biomass as a renewable energy source.
Biomass pyrolysis as an energy source. Source: image elaborated in powerpoint, images of wood and fire are of public domain.
The term biomass refers to all the organic matter that can be used to produce energy, and that remains accumulated in species and ecosystems. In a simple sense, biomass is all the matter that remains stored in a plant, and its oldest form of utilization is wood. Residual biomass is characterized by being heterogeneous, and can come from forestry and agricultural industries, such as nut shells, pruning residues, wood or sawdust.
However, in a more technical sense, biomass is more complex and is the matter that is made up of three components, cellulose, hemicellulose and lignin, and through thermal treatment the bonds of these molecules are broken and the energy accumulated in them is released, in addition to obtaining other value-added products, such as gaseous and liquid fuels, as well as coal.
And since matter of organic origin can be easily burned, all biomass is a potential source of energy, but, when waste is burned, there is usually no control over the generation of gases, ash and other types of combustion by-products, so only more waste is generated. However, the combustion of waste can be an efficient way to obtain energy and useful products, when it is carried out in a controlled manner through a process called pyrolysis.
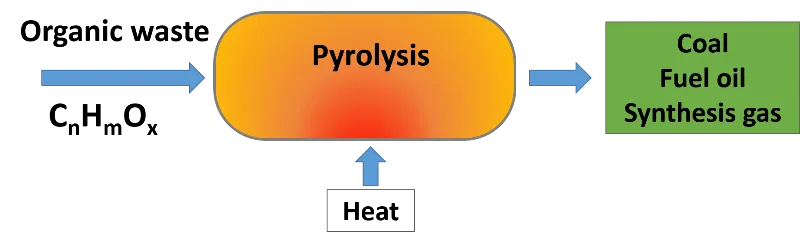
By means of the pyrolysis of biomass useful products are obtained. Source: @emiliomoron.
In pyrolysis the organic compounds, large and complex, are transformed under the action of high temperatures into much simpler and smaller products, some become gases, others become liquids and a part remains as carbon, in any case they are more stable and less polluting products that can be used as a way to generate energy. Therefore in this post we are going to make an introduction to the biomass pyrolysis process.
What is pyrolysis?
Pyrolysis is a thermal conversion process of organic matter in the absence of oxygen. It basically consists of the decomposition of waste under anaerobic conditions at temperatures ranging from 300°C to 800°C. The pyrolytic products obtained include a gaseous product (biogas), a liquid (bio-oil) and charcoal, with ash as an undesirable residue.

Biomass pyrolysis process in the laboratory. Source: @emiliomoron.
During pyrolysis the biomass is heated at a defined rate to a certain maximum temperature defined as the pyrolysis temperature, and the combustible residue remains there until the end of the process. The yield of each product depends on several factors, especially the composition of the treated biomass, the temperature and the heating rate. Low temperatures favor the production of liquid components while higher temperatures lead to higher production of gaseous compounds.
Coal production also depends on the pyrolysis temperature. Low temperatures lead to a higher amount of carbon, while higher temperatures lead to a lower amount.
This is why pyrolysis is currently used as a feasible process to transform different types of wastes and residues into higher value products, such as combustible liquids and solids that can be used to generate energy.
Types of pyrolysis
Pyrolysis can be a complex process, since it involves many chemical reactions and different factors. Usually the process is carried out under an inert atmosphere, which is usually N2, He or CO2 among others; in addition to the determined conditions of temperature and heating rate mentioned above. Depending on the operating conditions, the pyrolysis process can be divided into three subclasses: conventional pyrolysis, fast pyrolysis and flash pyrolysis.
Conventional pyrolysis: also known as slow pyrolysis, is the process in which low to medium temperatures are used, not exceeding 600 °C, with low heating rates so that they tend to have long residence times inside the reactor. This makes it possible to obtain solid, liquid and gas products in equivalent fractions.
Fast pyrolysis: In this process higher heating rates are used, the waste is usually heated rapidly inside the reactor in the absence of oxygen to temperatures above 600 °C, and the products are then cooled by means of a water stream. The high heating rates coupled with rapid cooling results in condensation of liquid fractions without cracking of the high molecular weight compounds, allowing for higher bio-oil yields.
Flash pyrolysis: is a type of ultra-fast process where higher temperatures (approximately 1000 °C) are used with shorter reactor residence times, allowing higher yields of gaseous products.
Pyrolysis products
Regardless of the type of pyrolysis, where one or another type of product is favored, in general the products of the process can be grouped into three groups:
- Biochar: this is the solid residue obtained, composed mainly of different forms of carbon.

Biochar obtained from biomass pyrolysis. Source: @emiliomoron.
The formation of this carbon is complex, but it can be schematized as follows:
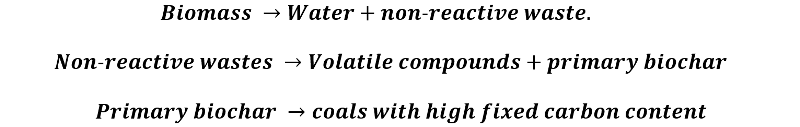
- Bio-oil: is a viscous liquid, usually very dark in color, composed of various organic compounds, including organic acids, esters and alcohols. Bio-oil produced by pyrolysis usually has a calorific value of about 17 MJ/kg.

Bio-oil from biomass pyrolysis. Source: @emiliomoron.
- Biogas: also called synthesis gas, it is a mixture of gases consisting mainly of CO and H2 (together 85%) with a small proportion of CO2 and CH4.
In conclusion
Biomass pyrolysis involves the thermal depomposition of waste plant material under anaerobic conditions, at temperatures ranging from 400 to 800 °C, with the main reaction products being biogas or synthesis gas, liquid products such as bio-oils and tar, and biochar as a solid residue. The yield of the process depends on several factors, mainly biomass composition, pyrolysis temperature and heating rate.
The main advantage of pyrolysis is that it is a feasible technology for the treatment and disposal of waste biomass, which can contribute to the reduction of environmental pollution by providing a better method of biomass disposal than furnace or open burning, where there is no control of the combustion products, while also providing a method for obtaining products with energy value.
Thank you very much for stopping by to read friends, I hope you liked the information and find it useful, see you next time!
References
Pooja Ghosh, Subhanjan Sengupta, Lakhveer Singh, Arunaditya Sahay (2020). Life cycle assessment of waste-to-bioenergy processes: a review. Bioreactors, Pag. 105-122.
Castro, Diana (2018). Evaluación del proceso de pirolisis aplicado al material lignocelulosico residual proveniente del pino en atmosfera de dióxido de carbono. Trabajo de grado, Facultad de Ingeniería, Universidad de Colombia.
Pinedo Urien Andrea (2013). Obtención de biocarbones y biocombustibles mediante pirolisis de biomasa residual. Trabajo de fin de master, Facultad de Ciencias, Universidad Nacional de Educación a Distancia.
Karthik Rajendran (2019). Influential Aspects in Waste Management Practices. Sustainable Resource Recovery and Zero Waste Approaches, Pag. 65-78.