
This article is a Steemit exclusive!
In the wake of beloved chef Anthony Bourdain’s recent suicide, some speculation has arisen regarding his prior use of Chantix, a smoking cessation drug manufactured by Pfizer.
Back in 2012, he tweeted about his use of the drug:
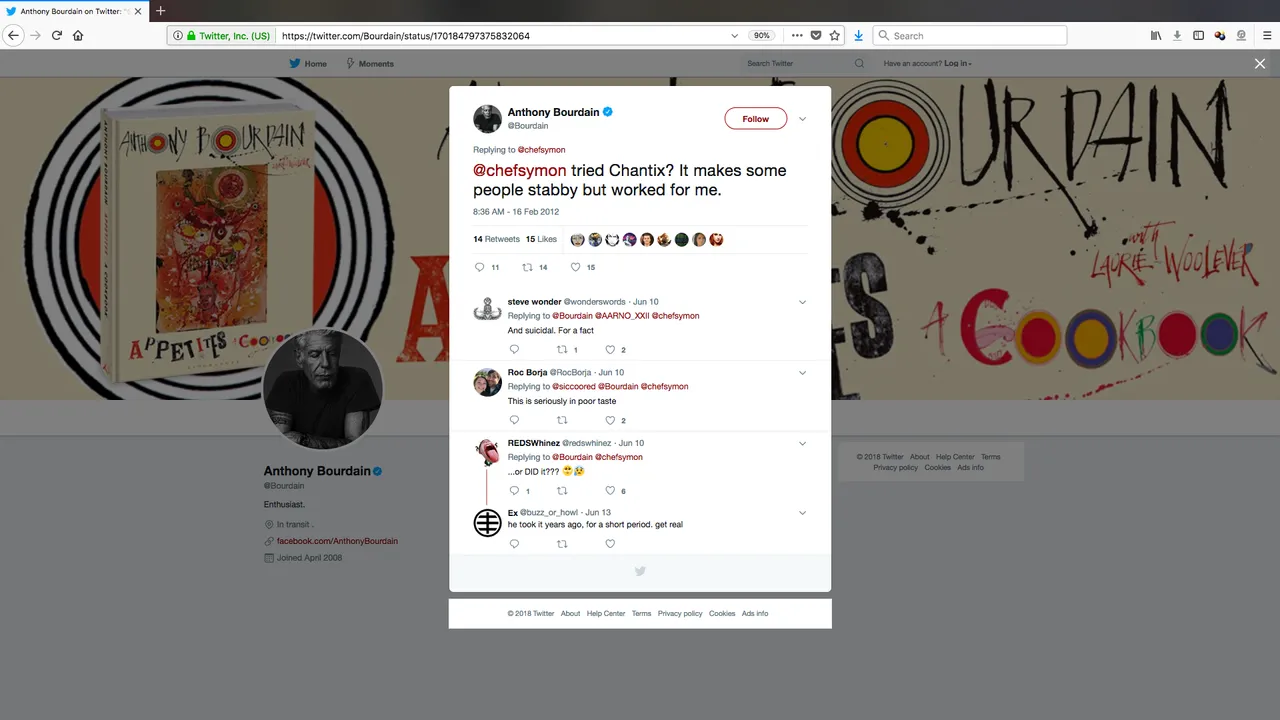
Chantix, generically called varenicline, has been widely associated with suicidal thoughts. In 2011, a report found it was eight times more likely to be linked with reported cases the risk of suicidal thoughts and depression than other anti-smoking treatments.
It’s impossible to realistically claim Bourdain’s experience with Chantix could be linked to his suicide considering there is no available evidence he used the drug recently, but there is a deeper, more systemic story at play.
In 2009, the FDA issued a black box warning for the drug due to its association with suicide and negative psychiatric side effects. In 2013, as many as 500 suicides and over 1,800 suicide attempts had been linked to the drug over the previous five years, according to a Freedom of Information Act request reportedly filed by Al Jazeera’s “America Tonight.”
Pfizer protested the black box label, citing “a meta-analysis of five studies and four observational studies showing its drug did not show an increase in suicidal activity,” as STAT News summarized it. But the FDA opted to wait for additional trial data and left the warning on the drug. That data, released in 2016, determined Chantix was not actually linked to suicidal thoughts.
The study, published in the Lancet, was produced by a group of researchers funded by Pfizer. Dr. Robert M. Anthenelli, an addiction psychiatrist and lead author of the study, received over $17,000 in 2015 from Pfizer to cover consulting and promotion services for Chantix. Neal Benowitz, an internist, received almost $10,000 in 2015 to consult on and promote the drug. Four other researchers in the survey were openly affiliated with Pfizer, and two others worked for GlaxoSmithKline, which manufactures a similar drug.
The study the FDA used to verify the drug’s safety was openly funded and commissioned by the company pushing for its approval even though the same drug was the focal point of over 2,000 lawsuits that led to the company paying $273 million to settle.
Though some scientists at the FDA questioned the validity of the company’s 2016 research, an “independent panel” ultimately rescinded the black box warning.
It is common practice for the FDA to accept research from drug companies as proof of their products’ safety, which should hardly come as a surprise considering the FDA’s well-established revolving door with the pharmaceutical industry.
However, as the problem of suicide continues to mount — and as evidence increasingly shows many government-approved drugs increase that risk — the FDA’s refusal to demand objective research into drugs it deems “safe” should be at the forefront of the conversation.
My Links:
Patreon: https://www.patreon.com/CareyWedler
Anti-Media: http://theantimedia.com/author/careyw1/
Youtube: https://www.youtube.com/channel/UCs84giQmEVI8NXXg78Fvk2g
Instagram: https://www.instagram.com/careywedler
Facebook: https://www.facebook.com/CareyWedler/
Twitter: https://twitter.com/careywedler