Producing clean hydrogen fuel has often depended on using freshwater and complex purification systems, making it both costly and resource-intensive. However, researchers from the University of Sharjah have introduced a novel approach that bypasses the need for desalination by generating hydrogen directly from seawater. This development marks a major step forward in sustainable energy, particularly for regions where freshwater is limited but seawater and sunlight are abundant.
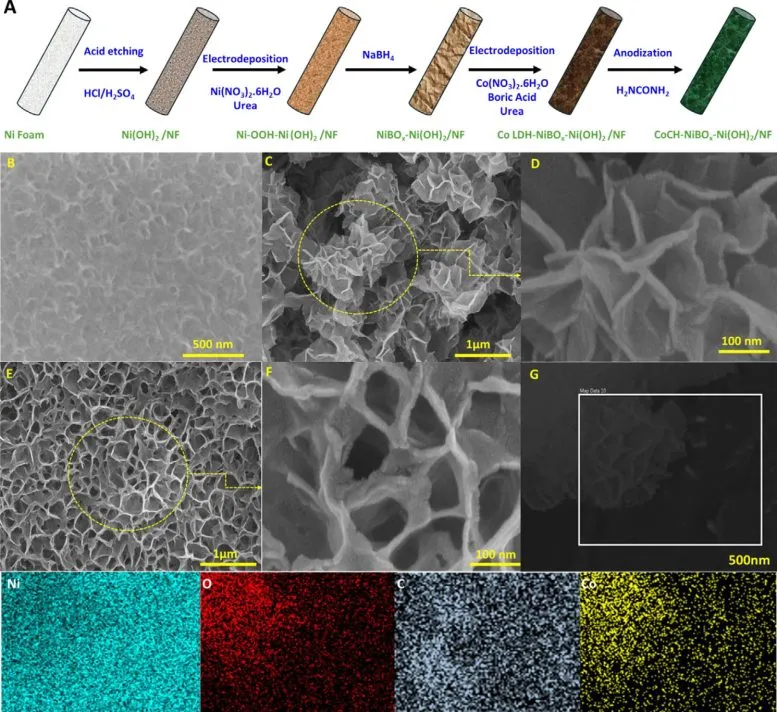
One of the biggest obstacles in using seawater for hydrogen production is the high concentration of chloride ions. These ions tend to corrode metal electrodes and reduce performance over time. The new approach, however, utilizes a specially engineered, multi-layered electrode that can operate efficiently in natural seawater without the need for chemical additives or pre-treatment processes. The electrode design not only maintains performance but also significantly resists corrosion.
At the heart of this technology is a carefully constructed electrode system embedded with carbonate ions and boron-enhanced materials. The structure includes a layered cobalt hydroxide base set within a nickel-based matrix. These materials work together to create a protective environment around the electrode. One critical feature is the formation of a metaborate layer that shields the electrode from metal degradation and prevents the buildup of non-conductive substances, which often hinder long-term performance in similar systems.
The design achieves an industrially significant current density of 1 ampere per square centimeter at a voltage of 1.65 volts under normal conditions. In layman’s terms, this means the system can generate hydrogen at a rate suitable for large-scale energy applications. Moreover, it does so with a Faradaic efficiency of 98%, indicating that nearly all of the electrical energy input is converted into hydrogen output with minimal waste.
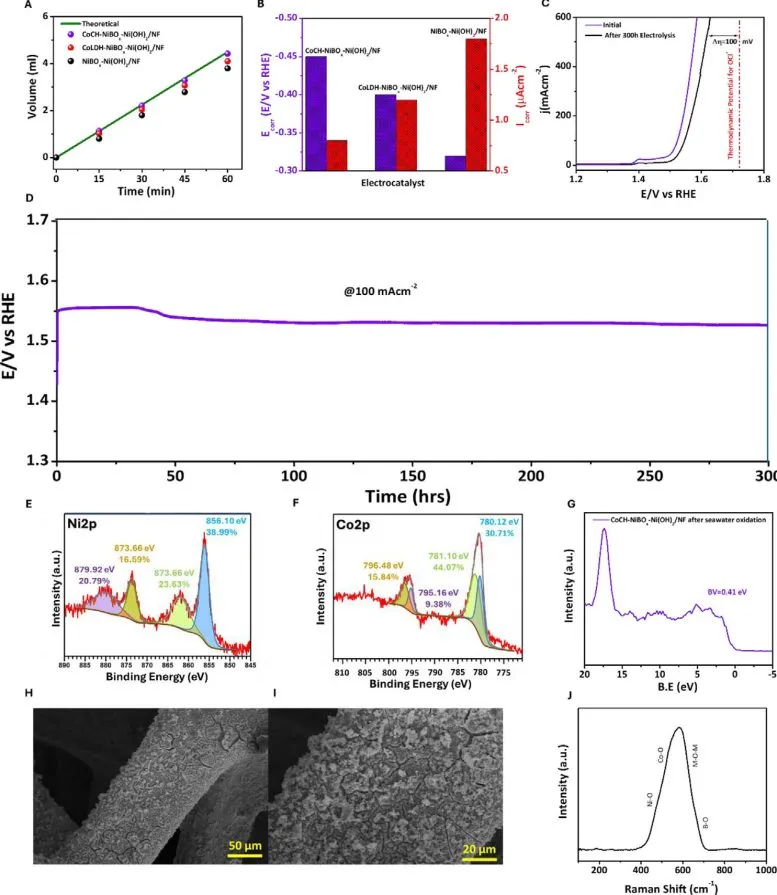
A key aspect of this system is its ability to create a unique microenvironment during the electrolysis process. The carbonate functional groups establish an acidic zone at the electrode’s surface, accelerating the reaction responsible for splitting water molecules into hydrogen and oxygen. This localized acidity helps counteract the negative effects of seawater’s chloride content, allowing the system to remain stable and effective over extended periods. In fact, tests have shown that the device can operate for more than 300 hours without significant performance loss.
This advancement is particularly promising for arid coastal areas like the UAE, where freshwater is scarce, but the environment provides ample sunlight and seawater. The technology could enable solar-powered hydrogen facilities along coastlines, converting readily available seawater into clean fuel without straining freshwater supplies or requiring large, expensive desalination plants.
One of the most exciting features of this innovation is its scalability. Unlike previous experimental models, this system has been proven to work under real seawater conditions and is now being adapted for larger pilot-scale applications. Researchers are working on modular designs that can be powered by solar panels and deployed in a variety of coastal settings.
The development holds the potential to reshape how hydrogen is produced, especially in regions that have traditionally faced barriers due to limited freshwater access. By transforming seawater into a reliable source of clean hydrogen, this technology offers an environmentally friendly alternative to conventional fuel sources. It also presents a cost-effective and sustainable pathway for nations looking to reduce carbon emissions and transition to greener energy systems.
As the global demand for clean energy continues to rise, innovations like this provide practical and scalable solutions. By making use of natural resources like seawater and solar energy, this breakthrough could help power the future without compromising ecological balance or depleting vital freshwater reserves. The research team at the University of Sharjah has not only solved a complex scientific challenge but also opened new possibilities for sustainable development in water-stressed regions.