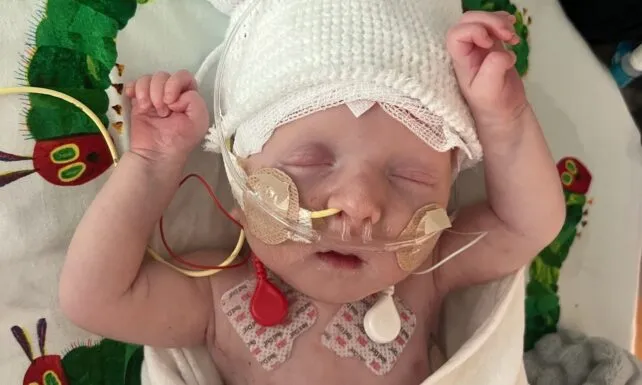
In a medical first, a groundbreaking approach to gene editing has shown success in treating a baby born with a life-threatening metabolic disorder. The patient, known as KJ, was diagnosed shortly after birth with CPS1 deficiency, a rare genetic condition that disrupts the body’s ability to process nitrogen. Without intervention, toxic levels of ammonia can accumulate in the body, causing serious brain and liver damage.
KJ’s condition meant he spent the earliest months of his life in intensive care, relying on a highly controlled diet and medications to manage his ammonia levels. However, a team of scientists and clinicians from the Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania developed a therapy tailored specifically to his genetic mutation—ushering in a new era of precision medicine.
Rather than relying on a one-size-fits-all gene therapy, researchers used CRISPR-based base editing to directly correct the unique mutation in KJ’s liver cells. The therapy was delivered using lipid nanoparticles, a cutting-edge technique that transports gene-editing tools into cells without the need to extract and reinfuse them, as is done in other gene-editing treatments.
This case is a departure from more standardized uses of CRISPR, like those recently approved to treat sickle-cell disease and beta thalassemia. In those conditions, cells are taken from the patient, edited in the lab, and returned. In KJ’s case, the therapeutic intervention happened directly inside his body. This bold step stemmed from years of laboratory research and collaboration, especially between Dr. Rebecca Ahrens-Nicklas of CHOP and Dr. Kiran Musunuru of Penn Medicine.
The team zeroed in on the most severe form of a urea cycle disorder—an area with limited treatment options beyond liver transplantation. Because liver transplants require a patient to be medically stable and old enough to tolerate surgery, the team’s goal was to intervene early and prevent the damage that ammonia buildup can cause in infancy.
By early 2025, the custom therapy was ready, and KJ received his first dose around six months of age. It marked the beginning of a cautious but hopeful process. He would receive two additional doses in the following months while doctors monitored his health, protein tolerance, and ammonia levels. Encouragingly, no serious side effects were observed, and KJ began showing signs of progress—tolerating more dietary protein and requiring fewer medications to control nitrogen in his system.
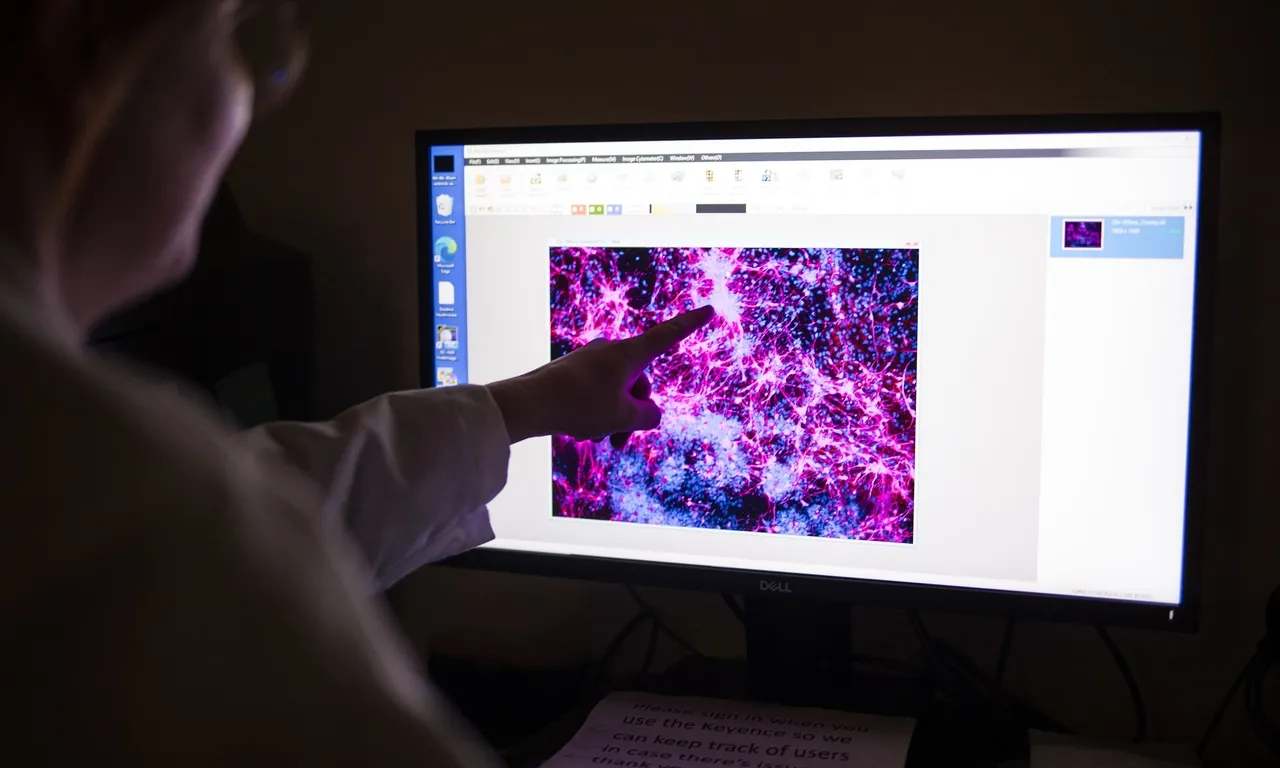
While the treatment’s long-term effectiveness remains under evaluation, early indicators suggest that KJ’s condition has improved more than expected for someone with such a severe form of the disease. This suggests that the gene editing may have altered enough liver cells to ease the symptoms and improve his metabolic function.
Though this therapy was custom-built for a single child, its success provides a powerful proof of concept. The researchers believe the same process could be adapted for other rare disorders caused by unique mutations. The work also hints at a broader shift in medicine, where gene-editing therapies can be developed rapidly and specifically for individuals who have run out of conventional options.
KJ’s parents, Nicole and Kyle Muldoon, say the experience has transformed their family’s life. From initially confronting grim prospects—including comfort care and potential lifelong disability—they now look forward to watching their son grow and thrive alongside his siblings.
This pioneering treatment was made possible through a coalition of academic and industry partners, including CHOP, Penn Medicine, and biotech companies contributing delivery systems and gene-editing components. Support from the National Institutes of Health’s Somatic Cell Genome Editing Consortium and other grant programs was critical in bringing the therapy to life.
While KJ’s journey is far from over, his case offers a powerful glimpse into the future of personalized medicine—a future where rare genetic conditions may no longer be a life sentence but a solvable challenge.