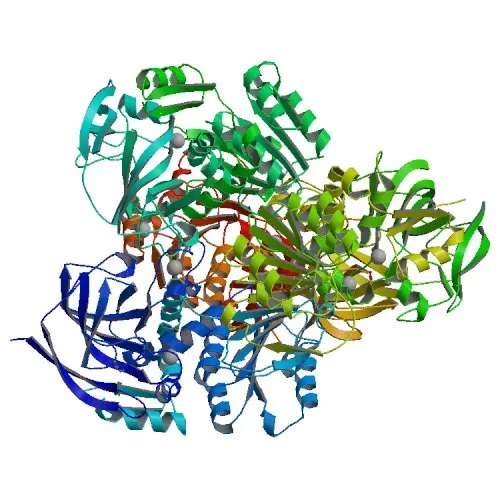
The study of proteins and their function is essential for understanding both cells and organisms. TM0436 is a putative alcohol dehydrogenase isolated from the hyperthermophilic bacteria Thermotoga maritima.
The study of alcohol dehydrogenases is important not only because these proteins are vital for cell metabolism, but also due to their wide range of potential commercial applications.
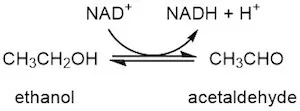
Alcohol Dehydrogenase action.
Alcohol dehydrogenases can be used in ethanol fuel cells to catalyze the transformation of fuel into electrical energy. These enzymes are often utilized in biotransformation processes as well, such as for the synthesis of enantiomerically pure stereoisomers of chiral alcohols. Furthermore, hyperthermophilic organisms produce proteins that have high stability, and are able to function under conditions that would inactivate or even denature their mesophilic counterparts.
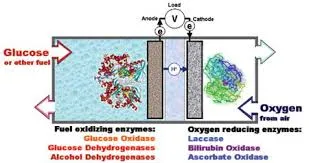
Enzymatic Biofuel Cell.
Evaluation of such proteins allows the correlation of structural properties with thermodynamic properties, and will allow for better engineered proteins. Enzymes used in fuel cells, biotranformation process, and therapeutic use could all benefit from such increased thermostability without the loss of activity. Thermotoga species have been used for bio-hydrogen production, biocatalysis in the food and paper industries, and transgenic expression in food plants, due to its well known thermostability.

Transgenic Expression of ADH in Tomato Plants.