I've only been active on this site for about 24 hours and I'm already addicted. Speaking of addiction, lets talk about making meth.
No, you won't be able to make meth after reading this; I've left out instrumentation, glassware, rxn setups, and other crucial data necessary to make the information I'm about to give you useful to the endeavor of making meth. It will, however, be a fun little romp in synthetic strategy that, at the risk of sounding arrogant, is just neat, and is something I'm rather proud of.
My fellow TA's and I used to play a game: we would pick an organic molecule and see who could come up with the quickest reaction pathway to it without using google. One day, we chose meth, and I did it in three steps, winning, thanks to some clever deductions.
First we brominate toluene, a simple process, if not an ideal yield.
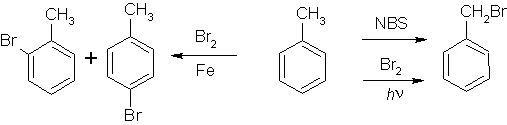
(Again, I'm not telling you how to do each step, so this data is useless without prior organic knowledge, which would be enough for you to figure out how to do this yourself. Anything to assuage my conscience)
Next, we're going to turn this into a grignard reagent. (Seriously guys, if you're still trying to use this post to make drugs, just stop. This step could kill you ezpz.)
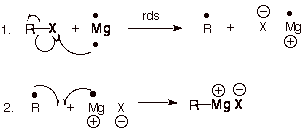
(our r group is the bromomethylbenzene from the prior step.)
So here's the cool part. I had the idea of reacting that grignard with the appropriate imine, followed by an acid workup, for the third and last step. I was met with some objection, as my friend Steph argued this was impossible. Her logic was that we could only go from nitrile to imine, or rather that nucleophilic addition to nitriles never produced amines in the same way that addition to carbonyl went to ethoxy or hydroxy bonds. Stumped, I thought about it for a second.
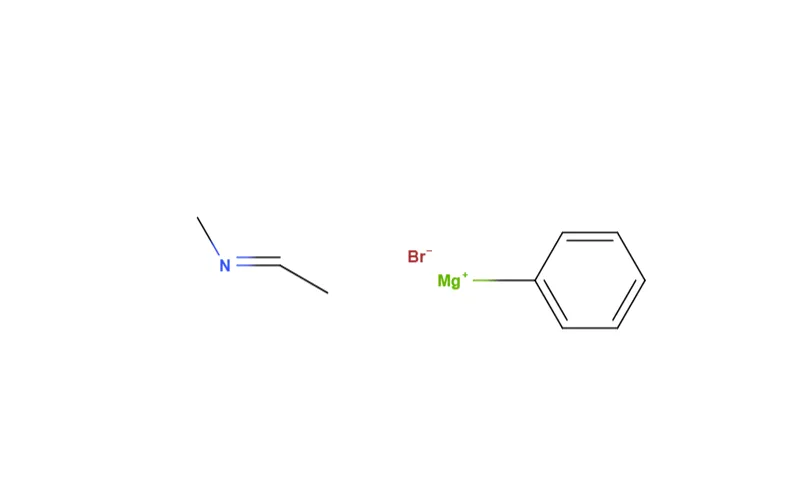
^imine and grignard
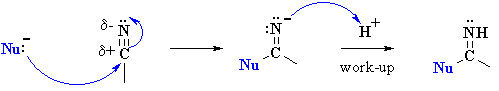
^stephs counterargument
After a moment or so of pondering, I stumbled upon something: in the nitrile example, an N-H bond would be formed while making the imine, rendering a grignard reagent impossible. Both R groups couldnt add simultaneously either; you can think about it as acid base chemistry, an N2- would be formed which has far too low a PKb to happen in the presence of what is effectively a deprotonated alkyl.
But,
In my example, we circumvent those issues, because there's no N-H bond on our imine, and only a N- is formed during the reaction, meaning it's a go.
There, that's how drugs made me smug for a day.
In all seriousness, I like this mechanism because it's simplicity. A common goal in organic synthesis is to shorten the pathway while attempting to keep percent yield at an acceptable ratio. For the most part this mechanism does that in gusto, and it's just fun to discover these kind of things.