Catenation
Organic compounds are the compounds consisting of hydrocarbons and their derivatives. The rest of the compounds are inorganic compounds. But, there are about 5 million organic compounds and just 100,000 inorganic compounds. There must be property due to which this fact is possible. And yeah, you guessed it right, its catenation property of Cabon.
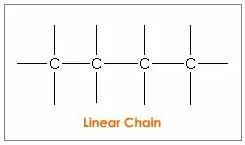
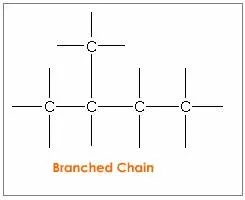
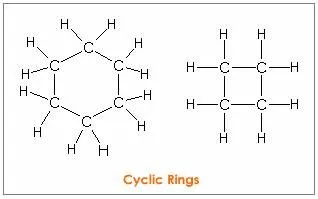
A carbon atom has a unique property of linking with other carbon atoms. This self-linking property of carbon is called catenation. A carbon atom can show tetravalency. It can be bonded to one two or three carbon atoms by forming single and multiple bonds. The link can be a linear chain, branched chain, or cyclic chain.
The catenation is carbon is due to a strong covalent bond between carbon atoms. The carbon-carbon bond enthalpy is large about 375 KJ/mol. That means 375 KJ of energy is required to break one mole of a carbon-carbon covalent bond. In contrast to this, Silicon-Silicon bond enthalpy is only about 220 KJ/mol.
Sources: