Hello friends, welcome again to another installment of my spectroscopy series.
But before starting the content of this publication I suggest you visit my previous deliveries:
In order to correctly follow the topic, we will ask the following questions and during the course of the publication I will answer each one of them. Below are the questions about the subject:
1. What is fluorescence?.
2. What is fluorescence spectroscopy ?.
3. How can we measure and how does a fluorescence spectrometer work?.
4. What can we obtain from a fluorescence spectrum?
5. What are your applications ?.
If you, my dear readers, have taken the time to review my previous deliveries, do you know what spectroscopy is? However, I will write the concept again.
Wikipedia tells us the following about the concept of spectroscopy:
Spectroscopy or spectroscopy is the study of the interaction between electromagnetic radiation and matter, with absorption or emission of radiant energy. It has applications in astronomy, physics, chemistry and biology, among other scientific disciplines.
While it is true that this study has a very large number of applications, it is important to know how we can perform this type of these experimental studies. Over the last few weeks I have been responsible for explaining each of the spectroscopy techniques that have currently served as the basis for the development of science. There are still many other techniques to be learned. The area of material science covers a lot of content. The characterization of materials when we refer to structural study covers many techniques with different studies among them, XRD and of course spectroscopy.
We have a lot of fabric to cut so we're going straight to the point.
Now, we are going to go into the matter referred to this publication, which is what really interests us at the moment. To answer the first question we can say the following:
1. We can affirm that Fluorescence is that process where an object or material emits visible light. This emission process is caused by electromagnetic radiation to then emit that energy in the form of electromagnetic radiation with a different wavelength. It should be noted that many people refer to the irradiation of ultraviolet light to obtain fluorescence, but that is not always the case!.
An example that I found in a book a long time ago when I was taking my undergraduate classes. He told us that a clear example of this situation is when we have a Rubrene plotter, this is activated by a green light in addition to the ultraviolet, which means that it is not necessarily necessary only ultraviolet light because sometimes it can emit or not light.
If we want to understand fluorescence, we should know a little about band theory, a very important subject for any scientist. What happens is the following:
"When light is absorbed by a material, the energy band of the electrons increases from one state to another, that is, from a lower level to a higher one with greater excitation." These electrons release all the energy absorbed necessary to emit light, but in a certain space they lose an amount of energy due to the vibrations that exist during this phenomenon, after they return to the initial state where everything started, these electrons emit light. "A clear example of how this process occurs is shown in the following figure.
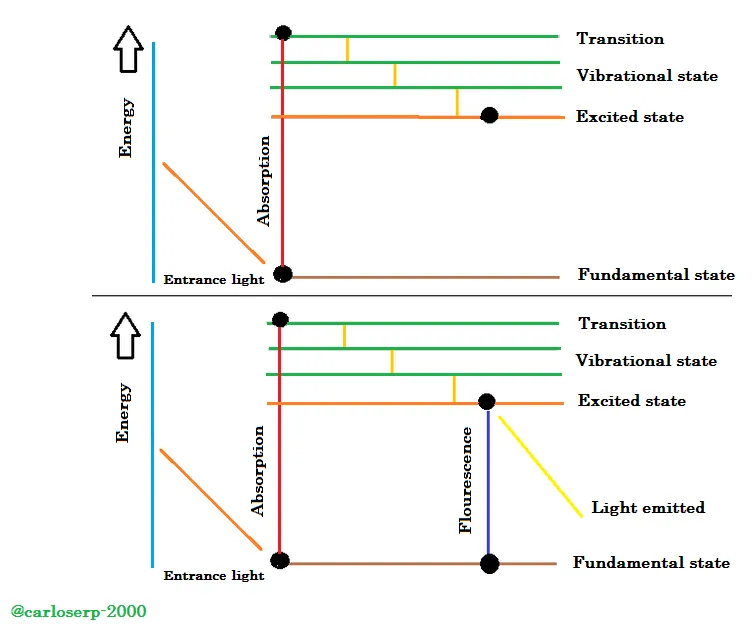
Fluorescence process
Now let's answer the second question: What is fluorescence spectroscopy?
2. First, we must have an object to which we will apply fluorescence, this material is excited through the absorption of a photon of light from a level or initial state in which it was at rest, called the basal state, to electronic levels where the electrons are found. excited. It could be said that fluorescence occurs in the middle of these two electronic levels as shown in the previous figure. Molecular collisions cause the molecule that is most excited to lose much of its vibrational energy, until it reaches a much lower level of vibration than the excited state.
When the molecule inside the material descends to the electronic levels of the initial state, it emits a photon during this process. It is important to know that molecules can be directed to very low levels of vibration in the initial or basal state, since photons have different energies and therefore different frequencies. And it is here where this type of study is focused because when obtaining by means of the analysis of the spectra the different frequencies of light emitted from the sample according to their corresponding intensities, it is possible to structurally determine the different vibration states or vibrational modes.
Let's answer the third question
2. If we want to obtain a fluorescence spectrum we must have the equipment indicated for this type of measurements, usually the following equipment should be used, the first one is the filter Fluorometers has the function of isolating the incident and fluorescent light through its filters. The spectrofluorometers use monochromators as all spectroscopy techniques, this has a particular diffraction graticule that helps the isolation of both fluorescent and incident light. All mentioned components are indispensable to be able to measure the fluorescence of a material.
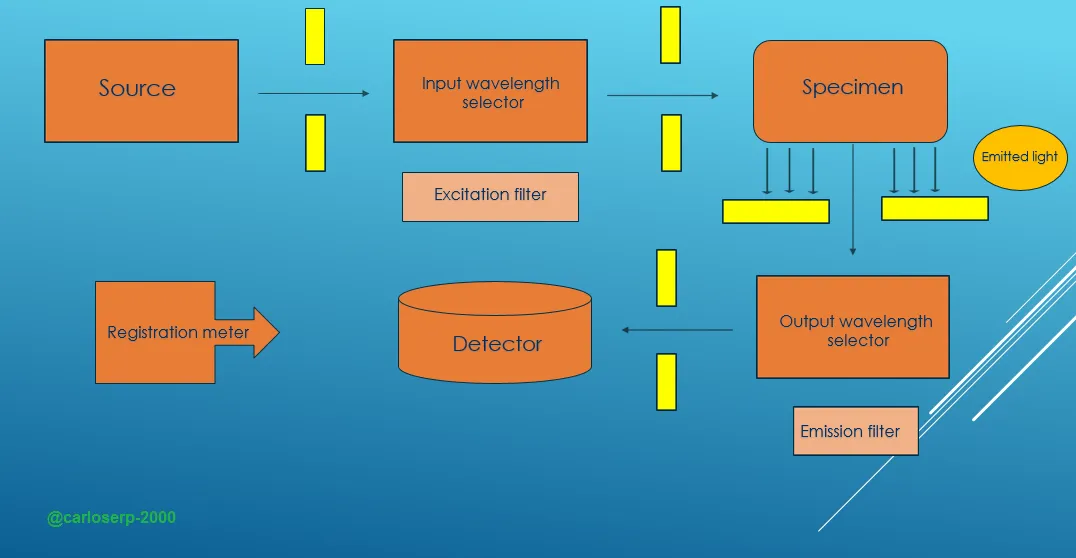
Experimental scheme of a fluorescence measurement equipment
Basically two types of configurations are used to obtain fluorescence spectra, first we must have available the most important tool for any spectroscopy equipment and it is a light source, in the same way an excitation wavelength selection system. When the sample is excited with radiation of appropriate energy it emits radiation in all directions of space. However, the emitted light is better detected at a right angle with respect to the excitation beam since light scattering problems and also the exciting light beam which is of much greater intensity than the emitted light beam are avoided. The emitted light is collected by selecting an appropriate wavelength and conducted to a detector where it is recorded by systems similar to those of an absorption spectrophotometer.
What can we get from a fluorescence spectrum?
4.Although to obtain fluorescence measurements is used more with the technique (x-ray fluorescence) through these spectra we can perform a very detailed quantitative and qualitative analysis of the concentrations of the material.
These analyzes can be performed perfectly with the technique of X-ray fluorescence, although I have not yet published an article on this subject I will explain briefly and later I will talk in depth about it.
Perhaps it is likely that we can ask ourselves the following question: then we are not deviating from spectroscopy, because we are talking about X-rays? in itself not ...! It is important to note that similarly through this method we obtain a spectrum and in detail is that I am focusing to answer the question I posed at the beginning of the post.
Through a fluorescence spectrum we can perform a quantitative and qualitative analysis, with the characteristic emission spectrum is quite simple and the emission of RX is given by the selection rules that are already defined through the theory of quantum mechanics.
The energy emission of the X-rays is transformed to a certain wavelength, which is unique for each element, as I have reiterated several times is its fingerprint, this allows us to emphasize the identification of the elements that are inside the material. When we use the energy or wavelength of the X-rays emitted for the identification of elements, the intensity of the X-rays allows the quantitative analysis.
We must emphasize that in the analysis the intensities are directly proportional to the concentrations of each element, that is, the more intense the emission or fluorescence, the greater the quantity of the element.
Standards are required for elemental quantification; that is, a certain element in some material at known concentrations. The standards are used to make a direct comparison with the sample to analyze. This is done by comparison with a calibration curve.
We must also point out the following:
At low concentrations, the fluorescence intensity will generally be proportional to the concentration of fluorophore. As opposed to 'standard' UV / visible spectroscopy, spectra indistinctly from the device are not easy to achieve. Several factors influence and distort the spectrum, and corrections are necessary to obtain true spectra, that is, spectra, obtained indistinctly from the device. The different types of distortions are going to be classified here as any instrument or relative of the sample. First, the root distortion of the instrument is discussed. To begin with, the light intensity of the source and wavelength characteristics vary with time during each experiment and between each experiment. In addition, the non-lamp has a constant intensity at all wavelengths. To correct this, a lightning dissociator called a monochromator or filter can be applied after the excitation, which is used to direct a portion of light to a reference detector.
This means that at the time of making the measurements in the first place the spectrometer must be calibrated well, an easy way is to obtain a spectrum already known and make comparisons with another spectrum and be able to observe if there is any distortion. And in turn it mentions us we must wait for an indicated time when making a measurement from one material to another, it could be said that it is a kind of rest of the instrument to avoid failures in subsequent measurements.
And finally we are going to answer the last question: ¿What are your applications?
Like many other techniques, fluorescence spectroscopy is not the exception because it has a wide variety of applications. It turns out to be very used for medical, chemical, biochemical analysis. In medicine it is applied in the identification and differentiation of malignant tumors of the benign ones. In turn in the identification of different organic compounds.
Fluorescence atomic spectroscopy techniques are practiced in other types of analysis, and measurement of a compound present in air, water or other medium, such as CVAFS that is used for the detection of heavy metals, such as mercury. It can also be used to re-direct photons.
And to conclude this iterative subject we can say that fluorescence spectroscopy has a great advantage with respect to absorption.
The first is that it has a sensitivity with orders of magnitude of 1 and 3, which in turn has a very large linear response interval.
Although fluorescence is less used than absorption due to the ability of some elements to fluoresce, it is very effective, however, it is also important to note that the equipment used for fluorescence spectroscopy is quite expensive.
If you want more information about the subject you can visit the following links:
10 Examples Of Electromagnetic Radiation In Everyday Life
Can we compute fluorescence spectrum of molecules like UV-Visible spectra, NMR and IR spectra?
Publish through our official app and you will get an extra vote of 5% https://www.steemstem.io/
 Video credits @gtg
Video credits @gtg