The cells in our body are in a constant state of homeostasis, which by definition is: "stable state of an organism and its internal environment". Survival means maintaining this stability for as long as possible. To achieve that, they need to keep certain parameters constant, such as temperature, but one that is usually overlooked is pH.
But what is pH in the first place?

pH Paper indicator - Source
pH can be defined as the concentration of Hydrogen ions (H+) in a certain solution or medium. Its value goes from 1 (acid medium, huge amount of H+ ions) to 14 (basic or alkalic medium, low amount of H+ ions). 7 means the medium is neutral, neither acidic or basic. Because of this, it is established that:
Acids = substances that give ions to the medium.
Bases = substances that take and bind ions from the medium.
The pH parameter is very important because, if it is too high or too low, it can affect the protein structure. In case of enzymes (proteic "speeders" of biochemical reactions) the pH instability can unable them, halting the reactions of the cell entirely and leading to its death.
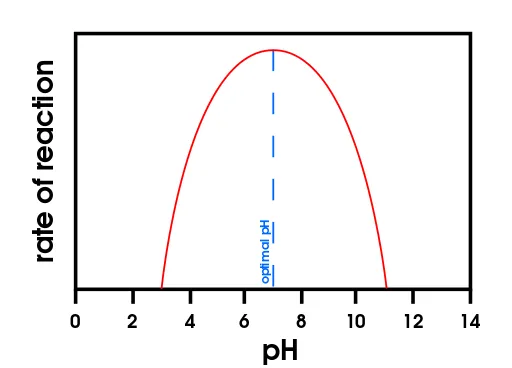
Optimal pH point for enzyme work rate of reaction. Note how when pH is below 3 or beyond 11 the rate of reaction drops to 0 - Source
Bearing this in mind, our body needs to maintain that acid-base balance for its survival. And the best way to spread that balance to the whole body is by the circulation of blood, which is stable at pH 7.4 (slightly basic).
Red blood cells in a vessel - Source
So, how does our body reach that precious balance of pH?
The human body is very smart and has 3 main mechanisms for its regulation: Buffer system, Respiratory regulation, Kidney regulation.
1 - Buffer System
It is a system which consists of a pair of substances in a reversible reaction (a reaction that can go backwards or forwards). This kind of reversible reactions are expressed with arrows in both directions, because the reaction will go in favour of the component that is in less amount. That is why they can be also called equilibrium reactions. In our cells it is composed of an acid and its conjugated base.
4 main buffers exist in our body: Carbonic, Hemoglobin, Protein, Phosphate
A) Carbonic buffer

This is the composition (reaction to the right) of carbonic acid (H2CO3) and decomposition of carbonic acid (reaction to the left) into carbonate (HCO3-, the base) plus one H+ ion.

This is the decomposition of carbonic acid (reaction to the right) into CO2 & water and (reaction to the left) composition of carbonic acid from CO2 & water.
As it can be seen from these chemical equations, the concentration of CO2, controlled by the regulatory respiration, is crucial for the amount of acid and base. Likewise, the concentration of carbonate, controlled by kidney regulation, is also important.
B) Hemoglobin
Hemoglobin (Hb) is the molecule that carries O2 and CO2 in blood. But it is also an important buffer component as its attached histidine residues (some proteic part) can donate or absorb 1 H+ ion from the medium. And hemoglobin can have up to 38 histidine residues per molecule!
Hemoglobin can also bind to O2 and CO2, and depending to which binds, its acid-base properties change in the following manner:
- Deoxyhemoglobin (Hb + CO2) tends to absorb H+ ions from the medium.
- Oxyhemoglobin (Hb + O2) tends to donate H+ ions to the medium.
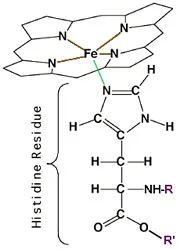
Hemoglobin (the big molecule) plus its histidine residue - Source
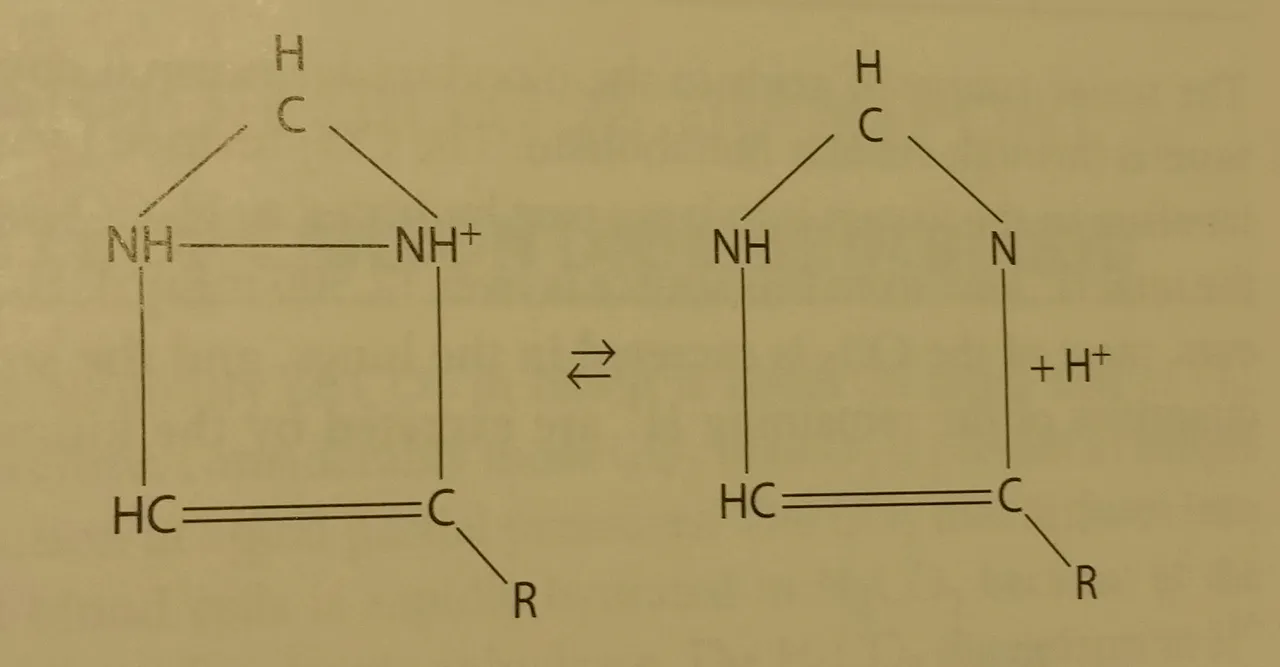
Imidazole (chemical compound) from the histidine group showing reversible acid-base reaction - Source from Physiology textbook (mentioned in the end)
C) Blood protein
All proteins by themselves have certain buffer capacity as they can donate or take one H+ ion from the medium, behaving as an acid or base respectively. In their optimal pH, the so-called isoelectric point, proteins are neither bases or acids. But they only have a tiny limited buffer capacity.
All proteins do this as all of them have an terminal -NH2 end that can absorb a H+ ion (transforming into -NH3+) & a terminal -COOH end, which could give an ion (and transforming into -COO-)
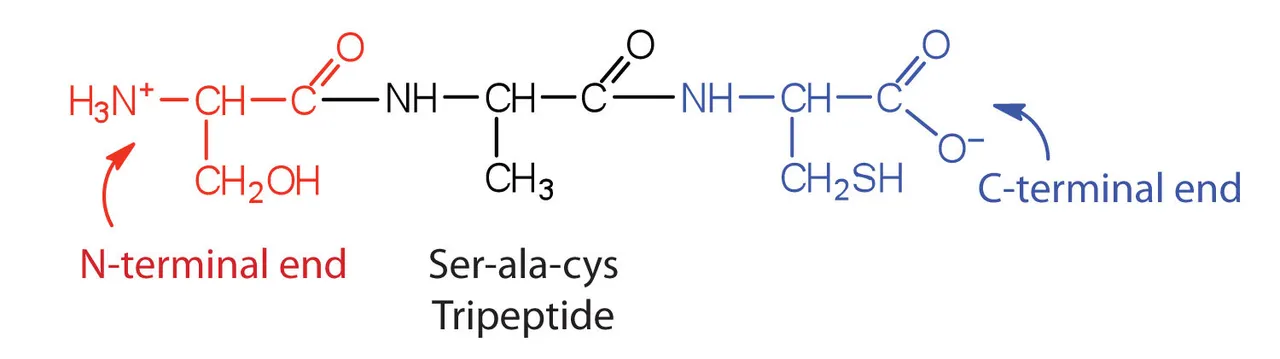
Tripeptide (Tri = 3, peptide = basic unit of a protein) showing the two ends - Source
D) Phosphate Buffer
Final buffer is phosphate which is mainly functional inside the cells. It is formed by dihydrophosphoric acid (H2PO4-) and its conjugated base hydrogenphosphoric acid (HPO4-2):
H2PO4- ⇌ H+ + HPO4-2
These are the forms that are more chemically stable and less harmful for the cells.
2 - Respiratory regulation
It is controlled by the lungs from the signals of one part of our nervous system just below our brain called Medulla Oblongata. It has a lot of sensivity to pH changes and to the Partial pressure of CO2 (or in other words, its concentration in blood). On its own it doesn't seem correlated, until we remember the carbonic buffer mentioned before:
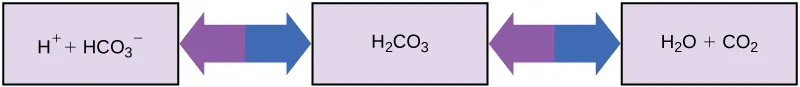
Entirety of carbonic acid balance, including its decomposition to CO2 and water - Source
If we are breathing less (because of an illness), there is an increase in CO2 in blood and with that increase, there is going to be a shift in the equilibrium equation to the left, to the creation of carbonic acid and its donation of the H+ ion.
On the other hand, if we breathe a lot more (let's say, while exercising), there is going to be a decrease of the amount of CO2. Thus, the equilibrium equation will shift to the right of the equation, to the consumption of carbonic acid to create CO2.
3 - Kidney regulation
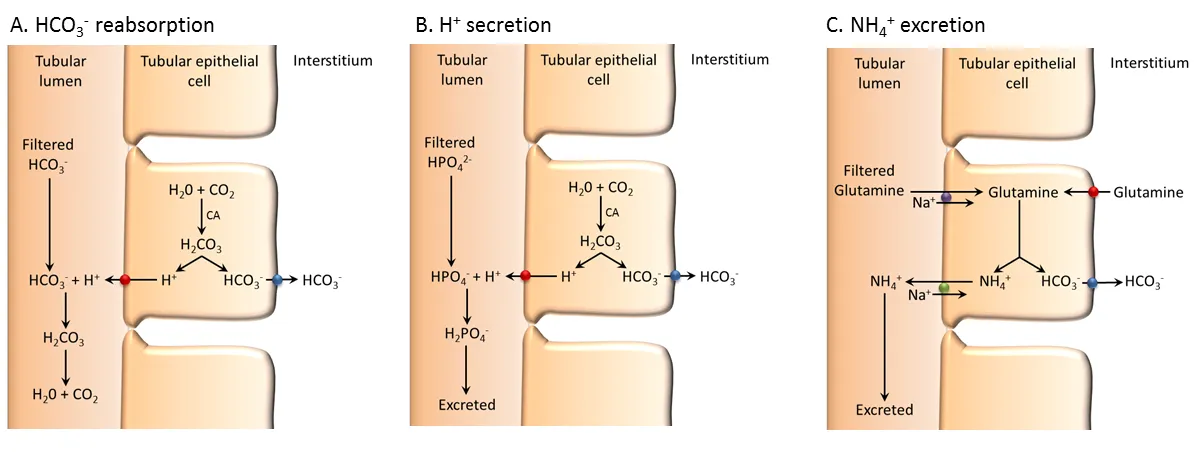
Renal regulation in the cells of kidney. Usually the 2 last steps are joined together - Source
Finally, kidneys filtrate the blood to reabsorb nutrients and excrete in urine unnecesary molecules. This is one of the last mechanisms of regulation as in-between those reabsorbed molecules are carbonate ions (HCO3-), which are going to be used in the above mentioned equilibrium equation for acid-base balance.
On the other hand, it is very important the excretion of unnecesary H+ ions in the form of ammonium (NH4+) following the next reaction:
H+ + NH3 ⇌ NH4+
That in term will form urea later, which will end up being urine.
References and source of images
Ganong's Review of Medical Physiology, 23rd Edition; Kim E. Barret, Susan M. Barman et al.
Acid-Base Homeostasis; L. Lee Hamm†, Nazih Nakhoul†, and Kathleen S. Hering-Smith† Link
Guyton and Hall textbook of medical physiology, 10th Edition; Hall, J. E., & Guyton, A. C.
Contributors of wikipedia in the wiki of Homeostasis https://en.wikipedia.org/wiki/Homeostasis#Blood_pH
Blood as a buffer from Chemistry-Libretexts https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Acids_and_Bases/Buffers/Blood_as_a_Buffer
Nataliescasebook http://www.nataliescasebook.com/tag/renal-acid-base-regulation
BBC.co.uk, GCSE website for Proteins and Enzymes http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa/proteins/proteinsrev1.shtml
Closing
Shoutout to @lesshorrible for giving me a starting point on my #introduceyourself to start publishing in #Steemstem! Thanks a lot!
This is @deholt, signing off!