The oil is separated into fractions by distillation and subjected to catalytic pyrolysis (cracking) in a refinery. The gasoline obtained directly from the oil fractionation (called direct distillation gasoline) contains mainly straight chain hydrocarbons and has an octane number around 50. This material undergoes a process called pyrolysis (cracking), which converts alkanes from a straight chain in other more desirable branched alkanes. Pyrolysis is also employed to convert part of the less volatile fraction of kerosene and fuel oil to lower molecular weight compounds that are suitable for use as automotive fuel.
Catalytic Cracking
Catalytic cracking is a process of oil refining that consists of the thermal decomposition of petroleum components in the presence of a catalyst, with the purpose of cracking heavy hydrocarbons whose boiling point is equal to or greater than 315 ° C, and convert them into light, short chain hydrocarbons whose boiling point is below 221 ° C. Said catalysts are presented in granular or microspherical form. Catalytic cracking produces naphthas and high-octane aromatic hydrocarbons, such as benzene through the conversion of cycloalkanes and paraffin.
Diagram of the Catalytic Cracking Process
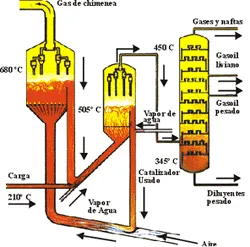
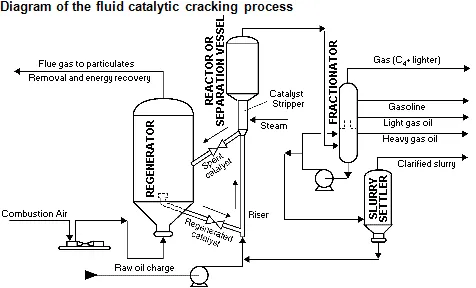
The catalyst heat vaporizes the feed and brings it to the desired temperature for the reaction. The reactions begin at the moment that the food. The basic process consists of preheating the fresh or recycled feed through exchangers and/or direct fire furnaces, then whole to the base of the riser of the reactor where it is mixed with the hot catalyst coming from the regenerator entanglement comes into contact with the catalyst hot in the riser, the vapors are subsequently separated from the catalyst in the reactor or separation vessel. The hydrocarbon vapors are sent to the fractionator to be separated into liquid and gaseous products.
In the regenerator, the coke burning is controlled by varying the air flow to maintain the reaction of the combustion gases. At the bottom of the fractionator, the crude oil comes out with the components that were not separated, making a recirculation to the column to which the temperature could be increased in such a way that the non-separated components can be volatile with said temperature increase, the same recirculation is obtained by another stream that goes to a sludge decanter producing clarified mud.
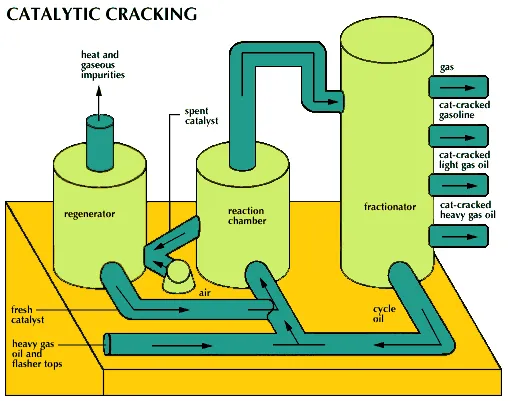
Catalytic cracking is a refinery process designed to produce more valuable gasoline out of less valuable, heavy hydrocarbon cuts. Straight-run heavy gas oil and flasher tops along with a catalyst are pumped into a high-temperature, moderate-pressure reaction chamber, where the conversion occurs. In the reaction chamber, coke (carbon) coats the catalyst and it becomes ineffective (spent). To remove the coke, the spent catalyst is put into a regenerator with hot air. The cracked cuts from the reaction chamber are pumped into a fractionator, where they are separated into cat-cracked gasoline, cat-cracked light gas oil, and cat-cracked heavy gas oil. Natural gas comes out of the top of the fractionator, and fractionator bottoms, or cycle oil, comes out the bottom. The cycle oil is run through the cat-cracking process again.