If you have been following my blog since the days of old.. well, if you can call one year ago the days of old! Then you will be familiar with the fact that I used to work mostly on DNA replication enzymes. As such, I spent a lot of time discussing various aspects of DNA replication, our genomes, genes, chromatin etc.
One such topic that was of particular interest to many people was a rapidly growing field known as epigenetics. The epigentics field is one which is centered on better understanding how DNA and protein modification processes, specifically one called methylation (addition of a methyl group -CH3) can influence how genes are expressed. I spent some time in the past discussing these concepts, so I won't belabor the point of discussing epigenetics in general.
Rather, today I would like to discuss with you a new publication from the journal Nature: Scientific Reports titled "Nucleosome stability measured in situ by automated quantitative imaging." In this publication the authors discuss their method for studying nucleosome structures that are so very important to both processes regulating our genes as well as to how our DNA is stored into chromosomes.

What Is A Nucleosome and Why Did You Bring Up Epigenetics In Your Intro?
Nucleosomes are protein complexes that are comprised of 8 subunit proteins called histones, specifically they are assembled from two copies each of four distinct proteins (H2A, H2B, H3 and H4). [2] Around these protein complexes, DNA spools like thread, completely encircling 1.67 times (Why not 2 times DNA? Huh? Why do you have to be so fractional!?). [3] This is the very first layer in the compaction process that turns loose free floating double stranded DNA into the tightly packed, very dense structures we call chromosomes!
Epigenetic modifications (again, methylation) come into play here, as whether or not a nucleosome is methylated determines how tightly the DNA gets wrapped around. When a nucleosome gets methylated, the interactions between it and the DNA become weaker, leading to the DNA being wrapped less tightly. This allows for RNA transcription machinery to gain access to genes that are on that DNA and begin to start the process of turning those genes into proteins.
Once Assembled Are Nucleosomes Like Biological Rocks? Or Do They Fall Apart?
To many of you, this is a question which may not seem particularly important to ask. However, let me assure you, it very much is! The stability of these nucleosome particles is essential to keeping our DNA compacted, however they can't be so stable that when our cells divide, our cellular DNA replication machinery gets stuck! In fact even the above mentioned RNA transcription processes are known to require regions of DNA that are free of any nucleosome particles. [4] It is also known that as the RNA polymerase gets access to DNA and transcribes genes from it, that it has to displace nucleosomes, even going so far as to remove parts of the histone octameric core (two pieces come out leaving a hexamer!) [5].
A lot of the information about how this all goes down was done on nucleosomes that were isolated from cells, or reconstructed from the individual histone subunits. [6] Researchers constructed artificial in vitro experiments using special fluorescently tagged histones, such that they could monitor these rearrangement processes, in many cases even in real time using single molecule microscopy techniques.[7]
Despite all of the detailed work done on these protein/DNA complexes in the past, quite a few aspects of how they function still elude researchers including the exact mechanisms behind how modification of specific locations on the nucleosomes regulate the stability of the nucleosome complexes themselves.[8]
To attempt to address some of these challenges the authors of this article wanted to create an assay that could be used to probe the nucleosome in situ (that means in its original position). They wanted to be able to enable researchers to be able to study the dynamics of these complexes directly in the cell, on the DNA, when they possess specific modifications.

The technique involves the use of high salt concentrations/compounds that intercalate in DNA (this means they actually stick themselves on top of the DNA bases), antibodies which specifically target a histone in the nucleosome core, and laser based microscopy (laser scanning cytometry). They called their technique Quantitative Imaging of Nuclei after Elution with Salt/Intercalators or QINESIn (a name so beautiful, I don't have a freekin clue how to pronounce it).
Things Will Now Focus On A Bit Of Detailed Science
For those of you who were interested in just learning a bit more about nucleosomes, please feel free jump on down to the conclusions/TL;DR section. I'll briefly wrap things up there.

For the remainder of you (I will assume there will be ... one? two?... zero? damn... :D ) let us dive just a bit into what the authors present their technique can do.
Probing Nucleosome Stability
If you are reading this, please comment the following "If it were legal, I'd marry a nucleosome", the first, and only the first person to comment this I will send 2 SBD as a prize for actually reading, and having the audacity to want to marry a nucleosome :p
Okay, so onto a little bit of data!
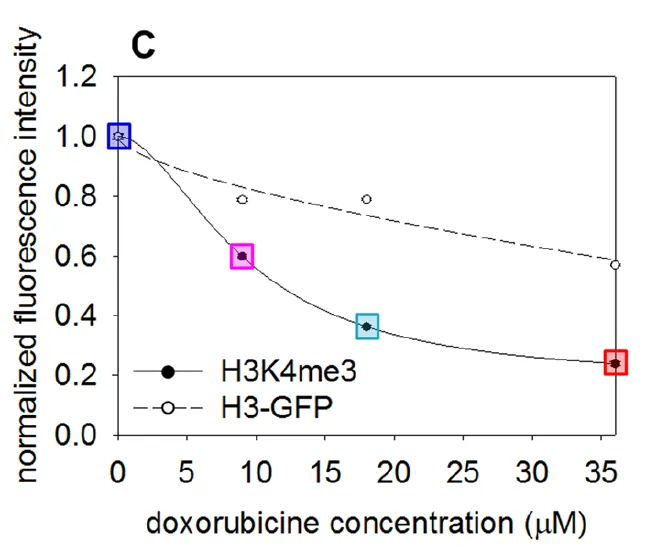
In the first experimental set described in this article the authors were repeating previous work which indicates that methylation of a specific lysine residue on histone 3 in the nucleosome leads to decreased stability of said nucleosome. So they utilized their technique of including an intercalating agent to kick nucleosomes off of DNA (they used doxorubicin). They then using an antibody specific for the nucleosomes and their laser microscopy method quantified the amount of nucleosomes that remained.
The figure to the left is a quantification of the relative fluorescence levels of non-methylated (H3-GFP) but fluorescencely labeled nucleosomes, vs methylated-nucleosomes (H3K4me3). What we can see is that as the concentration of the intercalating agent is increased the relative fluorescence from the methylated nucleosomes decreases significantly faster. This means that the methylated nucleosomes are less stable on DNA then un-methylated ones. This makes sense with our understanding of how nucleosomes function as if you recall from above, we discussed how the DNA becomes less tightly wound and genes more accessable when nucleosomes get methylated ( a method of epigenetic regulation ).
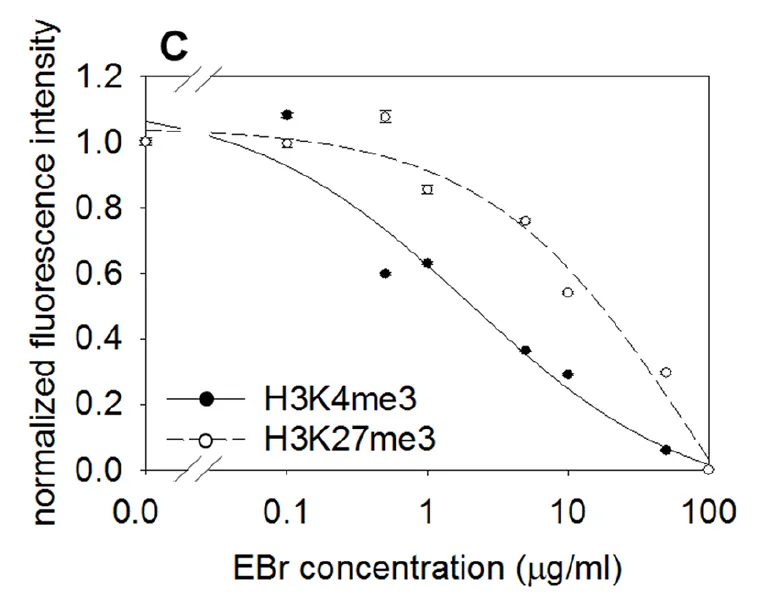
The authors also explored the use of other intercalating agents and methods to elute the nucleosomes. Here in the figure to the right, they were using increasing concentrations of the fluorescent intercalating agent ethidium bromide and have shown that there is a difference in the stability of nucleosomes depending on where the methylation modification is located. You can see that the fluorescence response from their assay decreases more quickly for the H3K4me3 nucleosome then it does for the H3K27me3 nucleosome. These experiments were done in HeLa immortalized ovarian cancer cells, however the result was not able to be repeated in two other types of cells (neural progenitor cells and mouse embryonic stem cells). In those, H3K4me3 and H3K27me3 appeared similar in their stability in the assay.
Conclusions/TL;DR/Wrap-Up
The authors of this paper have developed a slightly different way of monitoring the stability of nucleosomes on DNA/chromosomes and can achieve and visualize it in the nucleus of cells. This methodology can allow for more easy study of the complicated histone re-arrangement and nucleosome displacement processes which are necessary during both DNA replication and protein production processes in our cells.
There is a whole lot we don't yet understand about how DNA is compacted, about how these nucleosomal particles shuttle around, and about how epigenetic modifications (like histone methylation we discussed here today) effect these processes. Any new method to help simplify the study of these processes is badly needed due to the sheer complexity of the task!
The authors showed throughout this article (in more text and material that I did not even begin to discuss, as this post is already getting huge) that their method is able to effectively reproduce the results previously observed in the literature, potentially providing some level of comfort that this can be employed successfully by researchers going forward.
I feel like this post was a bit all over the place (the article was extremely information dense, and highly technical, so I really only touched on a very small subset of the data). However I hope that after having perused it you have left with a bit more of an understanding of nucleosomes, and perhaps a bit more of an appreciation of the incredible complexity that happens on and off our genetic material all so we can continue to exist.
Sources
Text Sources
- https://www.nature.com/articles/s41598-017-12608-9
- https://www.nature.com/scitable/definition/nucleosome-nucleosomes-30
- https://www.nature.com/nature/journal/v389/n6648/full/389251a0.html
- http://genesdev.cshlp.org/content/28/22/2492
- http://www.pnas.org/content/114/2/334
- http://pubs.acs.org/doi/abs/10.1021/jp7114737
- https://link.springer.com/article/10.1007%2Fs12551-016-0212-z
- https://www.nature.com/nrm/journal/v18/n9/full/nrm.2017.47.html
- http://www.sciencedirect.com/science/article/pii/S0014482799944774
Image Sources
All Non Cited Images Are From Pixabay.com, Flickr.com, Pexels.com, or Wikipedia.com And Are Available For Reuse Under Creative Commons Licenses
Any Gifs Are From Giphy.com and Are Also Available for Use Under Creative Commons Licences
If you like this work, please consider giving me a follow: @justtryme90. I am here to help spread scientific knowledge and break down primary publications in such a way so as to cut through the jargon and provide you the main conclusions in short (well compared to the original articles at least!) and easy to read posts.
SteemSTEM
Secondly, please consider supporting the @steemstem project. SteemSTEM is a community driven project which seeks to promote well written/informative Science Technology Engineering and Mathematics postings on Steemit. The project not only curates STEM posts on the platform through both voting and resteeming, but also re-distributes curation rewards as STEEM Power, to members of Steemit's growing scientific/tech community.
To learn more about the project please join us on steemit.chat (https://steemit.chat/channel/steemSTEM), we are always looking for people who want to help in our quest to increase the quality of STEM (and health) posts on our growing platform, and would love to hear from you!
