Today we will be discussing a recent publication in Nature: Scientific Reports titled "Engineering of Primary Human B cells with CRISPR/Cas9 Targeted Nuclease." I hope you will enjoy this summary, as we go a little bit through the background and material covered in this publication.
Human B Lymphocyte [Flickr.com]
B Cells and The Human Adaptive Immune System
This article focuses on the B Cell, aka B Lymphocyte, which is a cell involved in our adaptive immune response. When we say adaptive immune response we are referring to the type of response which is custom tailored (B cells secrete antibodies) to specifically target the invader. This is to contrast to our innate immune system which is not tailored to the specific invader but rather utilizes macrophages to attack any non-self material.
In a bit of un-intentional serendipity this article focuses on the application of CRISPR (the bacterial adaptive immune system) on those B Cells. However when people talk about CRISPR they are not really referring to CRISPR, but rather talking about the enzyme (RNA Guided Nuclease) Cas9 which is actually one piece of one variety of CRISPR.
What Is CRISPR Anyway: CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats, and actually refers to a bacterial genomic region where the organism stores pieces of foreign genomes (from viruses, or exogenous plasmids), the foreign DNA is stored in between repeating palindromic sequences of DNA, hence the name. This stored foreign DNA gets transcribed into RNA by the cell, and that RNA is used as a guide to direct the nuclease portion of the system to attack and cut up the foreign DNA (aka it functions like our immune system, but on the smaller bacterial scale, their invaders are viruses or DNA, so the CRISPR system uses the viruses own DNA as a way to attack it back, just as we use antibodies to identify our invaders and attack them back.)
Why Modify B Cells?
That's a fair question to ask. The main goal of modern medicine is to extend the human livespan as long as possible. One way to do that is to give our own bodies the correct tools to target bad material (eg. cancer) for more effective elimination. B cells play an integral role in our adaptive immune system functioning, as they are responsible for both presenting antigens (a marker for a foreign invader) and also producing antibodies, both of which "inform" T Cells who to go after and kill. Research has been done to make modifications to T-cells directly (the most advanced of those therapies is called CAR T and if you are interested in it, you can read a more generalized summary HERE) and these genetic modifications allow them to be more effective fighters against disease.
B cells are also attractive candidates for genetic modification and they have certain advantages over T cells. One advantage is that they are very easy to isolate, and grow very well in a lab setting. So it becomes possible to quickly and easily acquire and grow up very large quantities of cells. Were those cells then modified to express a particular antibody they could become a very effective tool for fighting against cancer.
Some Of The Authors' Work
In this paper the authors were trying to set up conditions necessary to allow for CRISPR manipulation of B Cells, so the work serves as a good example of what researchers need to do early on in the development of a technique such as this. The work isn't the most flashy, but I think this article serves as a great example of how basic science is pushed forward on a topic.
Establishing Growth Conditions For The B Cells
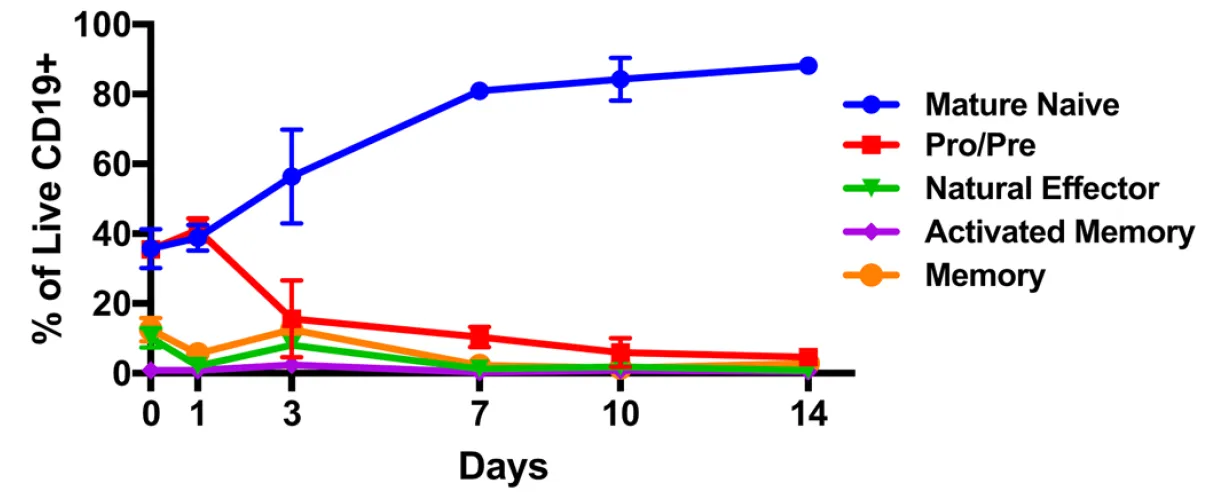
The researchers utilized a protein called CD40L which is a protein responsible for binding to a receptor on the surface of immune cells (both T and B) which results in increased rates of cell growth. Upon treatment of the cells with the CD40L protein, the types of cells in the culture were monitored and the respective proportions reported (as we see in the figure to the left). Here we see that the cells gradually grew to a composition which was about 80% mature native B cells.
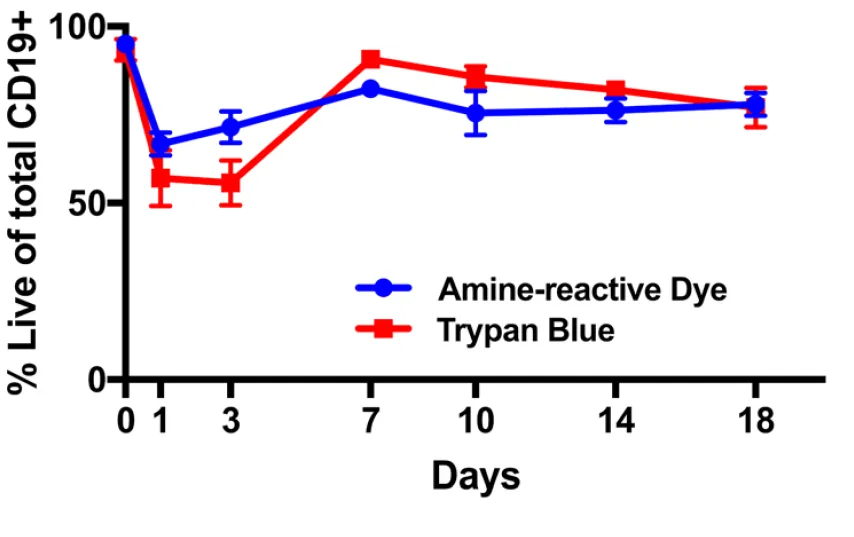
The authors also looked to see what proportion of the cells remained living. To accomplish this they used two dyes, an amine reactive die and trypan blue. Both of these dyes are only able to stain dead cells, living cells do not become colored. Through this they show that over the course of their experiment, their growth conditions result in about 85% living cells (which is good).
Getting DNA/RNA Into The Cells
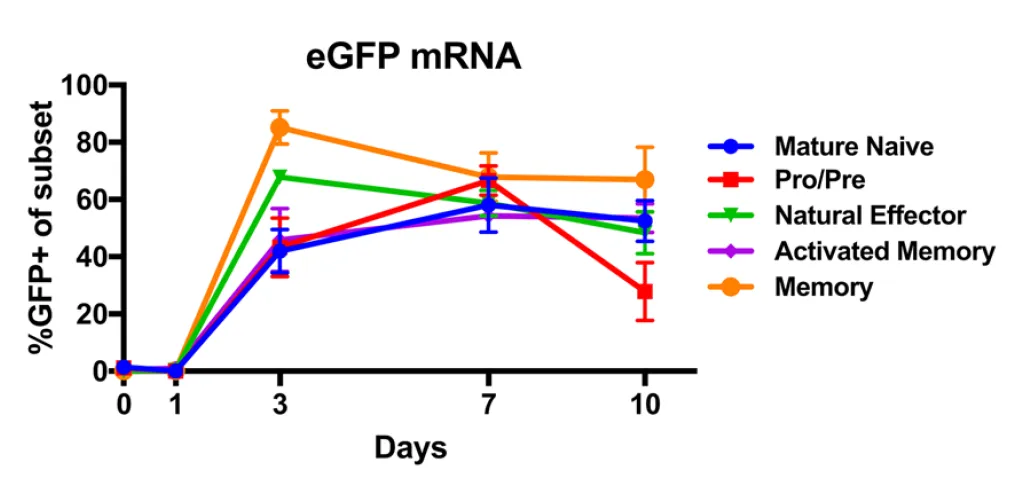
In order to make modifications to the B cells, one has to be able to get nucleic acids (DNA/RNA) into them. We do this through a process called electroporation where cells are zapped with high voltages (here the authors settled on three 10 milisecond pulses of 1400 volts), this weakens the cellular membranes (makes them more porous) and as a result some population of the cells may take up DNA that is in the media around them. Here the authors electroporated with mRNA containing a green fluorescent protein (GFP). They could then monitor the proportion of each variety of B Cell which turned green. We can see that after three days (but not before) in culture, the cells were receptive to the mRNA and it resulted in 40% or greater amounts of cells of each variety expressing the fluorescent protein.
Using CRISPR To Kill A Gene In B Cells
The researchers to this point have established effective culture, and DNA electroporation of the B Cells. Aka they can grow em, and they can get Cas9 and RNA guides in by their electroporation protocol. Now, can they use this to change the expression of a gene? To address this the authors targeted a surface receptor present on the B Cells called CD19.
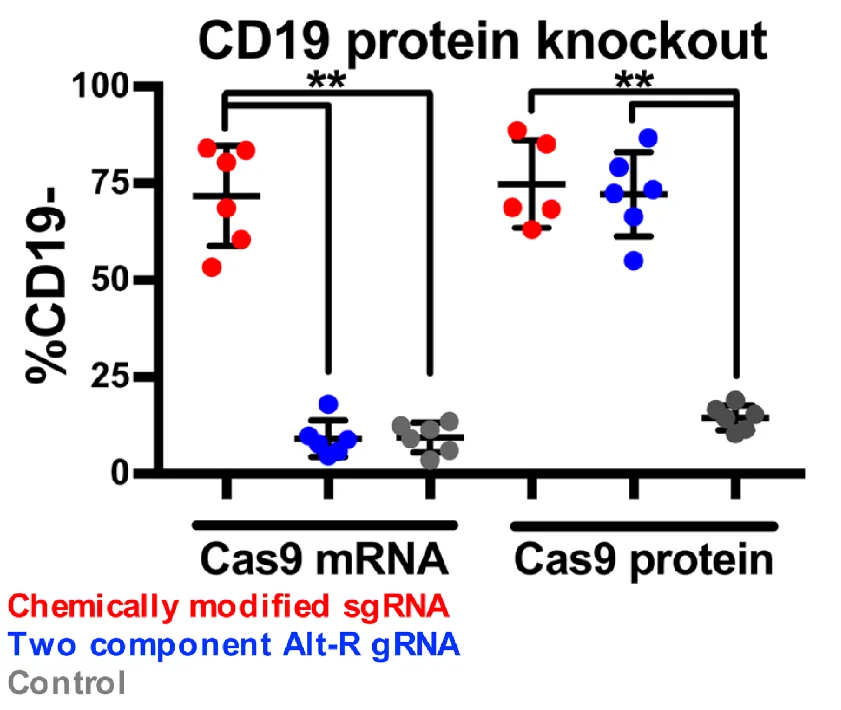
Here the authors were using fluorescently labeled antibodies to measure the amount of the CD19 cell surface receptor that was expressed after treatment with a few combinations of Cas9 and Guide RNA's. Here they used two different "styles" of guide RNA's and simultaneous or not transfection of Cas9. While I am not going to go into the nitty gritty about these two guide RNA types (chemically modified vs Alt-R). I have included some links to these two methods in case you would like to read more about what they entail.
What we see in the figure to the right, is that successful knockout of the CD19 gene occurs both with simultaneous transfected of the guide RNA and Cas9 nuclease and with electroporation of the guide and direct addition of the Cas9 protein. The authors note that better results were obtained (more cells lived) when using the chemically modified guide RNA and the cas9 protein rather then the cas9 mRNA, so this is the method they will move forward with.
Conclusions
The authors here have established protocols for expression, transfection and use of CRISPR to make edits in human B Cells. Potentially paving the way for an additional avenue of modified immune cell cancer therapies down the line.
- https://www.nature.com/articles/s41598-018-30358-0.pdf
- https://www.cancer.gov/about-cancer/treatment/research/car-t-cells