For ages, physicists have been trying to define time as precisely as possible. Today, the progress is such that one can define a second with a level of precision reaching 16 digits.
[image credits: Francis Flinch (public domain)]
This post is about time, and in particular about how physicists define time. @lamouthe (in her post here), @greenvago (in her post here; happy birthday, by the way ^^) and @rudyardcatling (in his comment to my posts) can now be happy ;)
Time is established from the International Atomic Time, which serves as a basis to setup the UTC time, i.e. the Coordinated Universal Time.
The latter is what regulates all clocks around our planet. It is thus damned important.
The TAI (and thus the UTC) is fixed according to atomic clocks, that are devices using some atomic properties to establish what is a second. Since the 1980s, a specific class of atomic clocks named atomic fountains are used and have allowed us to define a second at the 16-digit level.
This is the topic of this post, where I discuss how time is defined metrologically. And as a bonus, some atomic physics for free is available ;)
A SECOND IN THE EARLY DAYS (BEFORE THE 1960S)
But let us start with the beginning and focus on the second itself. A second is the unit of time included in the International System of units, abbreviated as SI (once again from the French version won).

[image credits: Pixabay (CC0)]
But this does not tell us what a second is. For that, please hold on for a second. I know: I ate a clown today and here is the proof.
In the ancient times, a second was defined relatively to the way our planet rotates. History indeed tells us that a second was defined as 1/86400 day before the 1960s.
This number is easy to get: we have 24 hours in a day, 60 seconds in an hour and 60 minutes in a second. The rest is a matter of multiplying numbers all together.
However, this is by far not precise! Earth rotation, which defines a day, is irregular. It show fluctuations here and there, and those fluctuations are bad for a standard. We need something better, more precise and especially more stable and uniform.
This is where the ephemeris time came into the game in the 1940s-1950s. Earth rotation around the sun is stabler that Earth rotation on itself, and metrologists from that time decided to move on with another definition of time.
The second hence became 1/31556925.9747 of the tropical year for 1900, this year being taken starting at January 0 at noon. And believe it or not, this is not the usual stupidity I am hiding in my posts. This is a real thing.
While this is used as a reference, something more practical appeared in the 1950s-1960s: atomic clocks. These devices were amazingly convenient as super stable and uniform. Natural players to become a new standard.
ATOMIC PHYSICS IN NUTSHELL
Before discussing atomic clocks (this will come in due time… the clown is back), let us focus for a second (again) on atoms, and in particular on how atoms are made.
Atoms are made of a core, the atomic nucleus determining the atomic species, and a bunch of electrons orbiting around it. An illustration is shown below with a cesium atom.
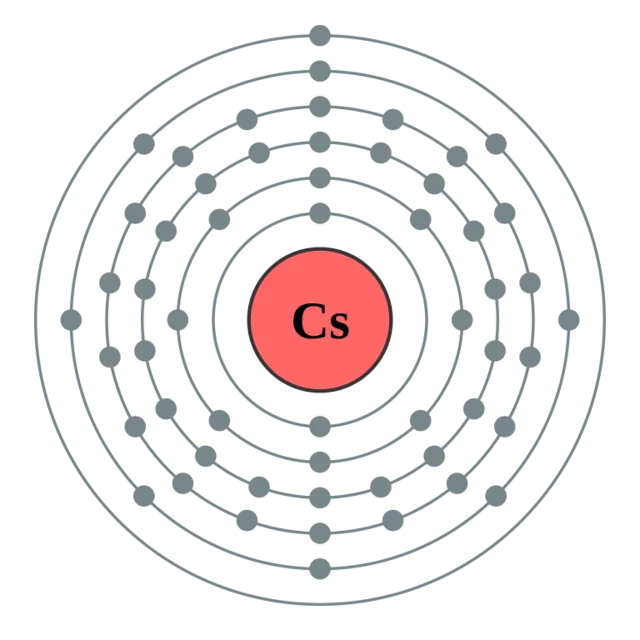
[image credits: DePiep (CC-SA 2.0)]
We have here a core made of 55 protons and neutrons, and 55 electrons around it.
The electrons cannot however be randomly distributed around the nucleus, and they fill what are called electron shells.
On the picture, each line around the nucleus is a shell, and it can receive a well-defined maximum number of electrons.
The fundamental level of the atom, for which the energy is minimum, is the one in which we start filling the shell that is the closest to the nucleus, then moves with the next-to-closest, and so on until we have added all the 55 electrons.
In order to take one of the electrons and move it away, in a more outer shell, one needs to provide the atom some energy, bringing it in a so-called excited state. Inversely, an excited atom will naturally move back to its fundamental state by emitting some energy.
Quantum mechanics however tells us exactly how much energy is involved in a specific transition, and that this value is unique.
ATOMIC CLOCKS
Atomic clocks use this principle in a very simple manner.

[image credits: Zubro (CC-SA 3.0)]
One starts with a bunch of atoms prepared in a state of given energy E, and we bombard them with some microwave photons in order to bring them in a targeted excited state of energy E’.
As we know both energies E and E’, we also know the energy needed by the photons.
I recall that for photons, energy and frequency are proportional to each other, as detailed here.
We thus only need to generate photons at the right frequency to allow for the E to E’ transition at a huge rate.
By counting the number of atoms of energy E’ that we get, one can tune the photon energy (or frequency): The closest the photon energy will be from the E-E’ difference, the largest amount of atoms of energy E’ we will get. Easy!
In other words, we have an oscillator, and the idea is to tune its frequency to the frequency of the atomic transitions. By connecting an electronic circuit to the oscillator, we obtain a clock.
Using cesium atoms, the right frequency is 9192631770 Hz. And since a second is the inverse of a Hertz, here is our definition, which lasts since 1967.
ATOMIC FOUNTAINS
In the 1980s, a new type of atomic clocks was designed: the so-called atomic fountains.
One of the major issue with the performances of the atomic clocks is connected to the speed of the atoms. If they are cold, they move slowly and physicists have time (yeah… again) to compare the frequency of the oscillator with the one of the atom.
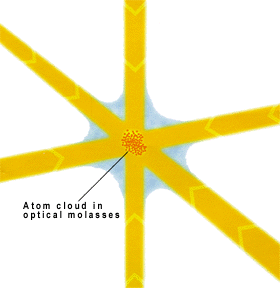
[image credits: Andreas Lilius (CC BY-SA 3.0)]
In order to slow down the cesium atoms and cool them down, the idea is to bombard them with a bunch of lasers with the right frequencies, as depicted in the picture on the left.
The area where the lasers cross consists a kind of viscous medium where the atoms are kind of glued. They are slowed down very efficiently.
In other words, they are cooled down to temperatures very close to the absolute zero. In fact, just a billionth of degree above it.
After being cooled down, the atom are thrown upward, at an altitude of about a meter, and they then fall by gravity. The setup is called an atomic fountain. I think the name speaks for itself. We should just imagine many many atoms.
Metrologists then probe the properties of the atoms during its rise and its fall.
They have managed in this way to improve the precision of the clocks by a factor of about 100. In other words, a leap second has to be inserted once every 300 million years!
This is precision, isn’t it!
SUMMARY
In this post, I browsed how time was defined by physicists. Starting with the early days using the motion of Earth, I moved on with the atomic clocks and fountains using the cesium energy levels to define a second.
The idea behind that is to bombard cesium atoms with photons of a given energy and to tune the frequency of the photons so that a maximum of atoms will undergo a specific electromagnetic transition. The tuned frequency is then the one used for defining a second, as we theoretically know what it is.
Thus being said, better clocks exist today, using different atoms. Targeting transition with much larger frequencies, one can then easily reach precision and stability of 16 to 18 digits. For instance, strontrium or mercury, etc.
A stupid stuff is hidden in this post… Can you find it? And it is better hidden than usual ;)
STEEMSTEM
SteemSTEM is a community-driven project that now runs on Steem for more than 1.5 year. We seek to build a community of science lovers and to make the Steem blockchain a better place for Science Technology Engineering and Mathematics (STEM).
More information can be found on the @steemstem blog, on our discord server and in our last project report. Please also have a look on this post for what concerns the building of our community.