Hi everyone, I hope this finds you well. Thank you all for the support and advice you gave me via your lovely comments on my previous post. Today, let's take a look at various types of separating techniques, what they separate and how they separate mixtures.

A mixture is one whose constituents are not chemically bonded. All mixtures are either homogeneous or heterogeneous. Homogeneous as in they have uniform composition, what do I mean? Take air for example, the air you will find in the Buckingham Palace has the same composition as that you will find in the suburbs of Lagos. Air, we know, contains a fixed amount of nitrogen, oxygen and other elements. Air is the same all over the world that makes it a homogeneous mixture. I guess we all know what a heterogeneous mixture is now.
We often need to separate mixtures and there are a number of techniques that can be used.
Examples of separating techniques includes Crystallization, Filtration, Decantation, Sublimation Evaporation, Simple distillation, Fractional distillation, Chromatography.
Picking the best method to separate mixtures is totally dependent on the physical properties of the constituents of the mixture. Properties like boiling and melting points, magnetic property, density, solubility etc.
Let's take a look at some of the various separation techniques beginning with Evaporation.

Evaporation and Crystallization
Some years back while I was in elementary school, my big brother came back from school and said he would like to show me a magic trick. For the trick, he said he needed table salt and water. He made me pour table salt into water and stir until the salt no long appeared. “What is this magic trick?” I asked. He told me he was going to bring back the salt I could no longer see in the water. I laughed so hard and told him to amaze me.
He poured the salt-water solution into a pot and started to heat it on the stove. Down went the fuel in the stove, up went water vapours. I made sure he didn't stand up to do anything that would trick me. After a while, there were no visible water vapours going up. He asked me to go check inside pot, I stood up and walked to the pot. Inside was this white substance I was trying to convince myself isn't the salt I put inside water. I looked at my brother the same way Dorothy looked at the great wizard of Oz.
It was only later I learnt in school that the magic trick was actually a separation technique called evaporation. Evaporation is the continuous escape of liquid molecules in vapour form from the surface of a liquid. This was the trick my brother employed - heating and driving off the solvent solvent (water) leaving the solute (salt) as residue. Personally, I view evaporation as a recovery technique more than a separation technique because you are only getting one part of the solution. Evaporation is not ideal in recovering solutes which are prone to decomposition on heating, which leads to my next technique.
Crystallization separating technique use the same method evaporation uses. The only difference between them is that crystallization can recover solutes that decomposes on heating. Unlike evaporation where the ratio of solvent to solute does not really matter. In crystallization, the solution must be saturated. But when is a solution said to be saturated? Imagine a parking lot with cars taking up the whole space. New cars can't come in at that particular time, then we say the parking lot is saturated. Saturated solution contains the maximum amount of solute dissolved in a solvent at a particular temperature.
To separate by crystallization, you have to raise the temperature of the saturated solution and allow it cool down conventionally. As the temperature reduces, solubility decreases also thereby allowing the excess solute to crystal out. The crystals are then separated from the solution using another separating technique called filtration. Example of a solid solute that decomposes on heating is copper sulphate salt.

Filtration and Sieving
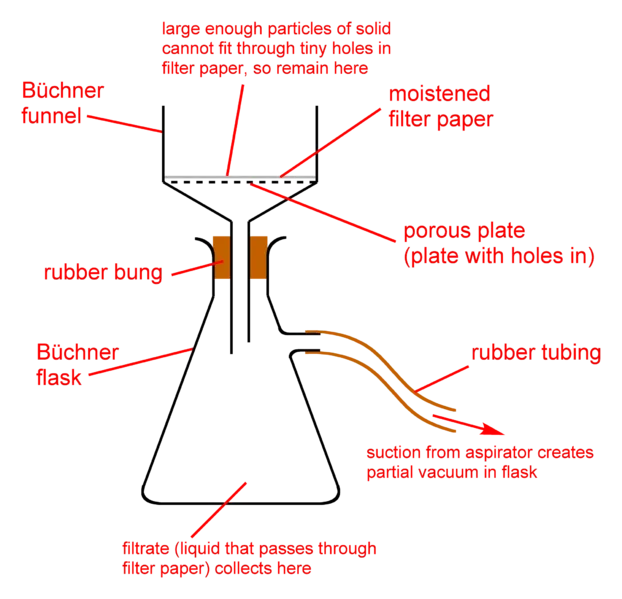
The previous two methods I discussed separates a soluble solid from liquid. Filtration however separates a non soluble solid from liquid. I guess the main reason why it is called filtration is because it uses a filter. No, it's not instagram or snapchat filter in case you are wondering. It's a filter paper.
A filter paper has little holes in it, big enough to allow liquids pass through it and small enough to stop solids of even the smallest size passing through it. The filter paper is folded into a cone shape and is put over a fitting container. The solid-liquid solution is now poured into the coned filter paper with the liquid making its way down into the container and the filter paper holding unto the filtered solid. Should you be asked what is the name of the liquid in the container, don't hesitate to let the person know it's called a filtrate.
Sieving is simply using a sieve to separate a solid from a solid. What the hell am I talking about? When you have a mixture of solids which are of different sizes, instead of separating them by picking out the bigger or smaller of the mixture, you can find an appropriate sieve to pour the mixture through and you will be sure to have them separated.
If you noticed, I said appropriate sieve. What I mean is basically a sieve that has holes small enough to allow the smaller of the mixture pass through it and big enough to retain the bigger solid. This finds application in construction sites as they look to separate stones from sand.

Distillation
If you remember I mentioned earlier that physical properties play an important role in determining the method of separation. Distillation is the separating of a mixture of two or more miscible liquids. Miscible as in they dissolve in each other. For example, adding juice to water will give you a perfect solution as you won't be able to point out an area where water is more than the juice or vice versa. There are two types of distillation, namely Simple and Fractional distillation.
The process of distillation is very similar to evaporation. Remember I said during the magic show my brother put on for me, we watched the vapours go up the sky. For the distillation process the vapours are not allowed to escape into the atmosphere.
Distillation occurs in a distillation flask which has a sealed top. The mixture of the liquids is poured into the flask and heat is applied. The liquid with the lower boiling point starts to boil and it's vapours go up the flask where there's an outlet.
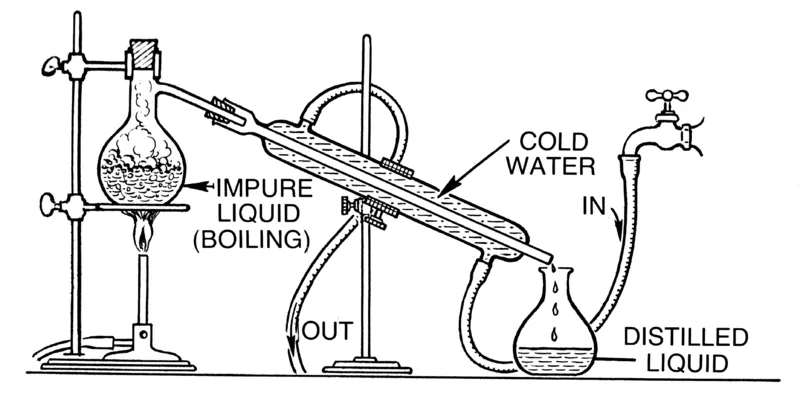
This outlet is connected to a condenser which is also connected to a water source. As the vapour enters the condensing tube, water from the water source circulates around the outside of the tube and then in turn takes away some heat from the vapour. After heat is taken from the vapour, it changes its state back to liquid. With the condenser slanted downwards, the condensed liquid trickles down into an open flask.
The major difference between simple distillation and fractional distillation is the difference in boiling point of the two liquids. For simple distillation, the difference between the boiling point of the liquids must be of a significant value while the difference in boiling point must be sufficiently close for fractional distillation.

Separating funnel
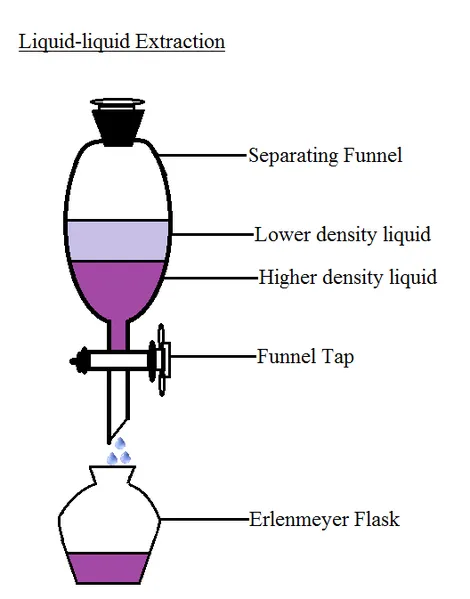
If distillation separates miscible liquids, I'm sure you definitely want to find out what separates immiscible liquids. A separating funnel does. Immiscible liquids are those that don't dissolve in each other. A typical example is the oil and water. The physical property that play an important role here is density.
A separating funnel is a laboratory glassware, since we are using water and oil as an example. Water and oil mixture is poured into the separating funnel and is shaken vigorously and is allowed to settle. The liquid with the higher density of the two sinks to the bottom while the other floats on it. When you were a kid and you want to take a taxi with your elder brother and there's only a seat. He makes you sit down on his laps, right? Why? Because he's heavier than you are. You can think that mixtures too would do the same. The heavier of them allows the lighter one to float on top of it. There a tap at the bottom of the separating funnel which when the mixture settle can be opened slowly to allow the liquid at the bottom out slowly while the other is stays in the separating funnel.

What happens when you have a mixture of two solids in which they are of the same size so sieving can't work? Provided that one of the solids can sublime i.e. go from solid to gas, sublimation will help separate them. The mixture is placed in a funnel and heated. The application of heat to the mixture causes the solid that can sublime (example of such is iodine crystals) changes state and turns to gas. A cotton wool can be placed over the funnel to absorb the evolved gas.

Importance of separating techniques
You may have been wondering of what importance is separating mixtures or where does separation of mixtures find its application.
Table Salt is a basic and very important ingredient used in cooking. Table salt is just sodium and chlorine. But, do you think table salt is produced by combining sodium and chlorine in the laboratory. Of course you will get salt, but the cost of production will be on the high side and the goal of any production is to produce at the barest minimum cost.
So, how do we get common salt? If you have ever swam in an ocean or sea. You will know seawater is salty. Seawater is collected and heated to dryness which leaves salt.
The refinery process of crude oil through which we get different products like natural gas, kerosene, asphalt, lubricant and fuel oils is as a result of distillation of crude oil.
The importance of separation techniques can not be overemphasized as it finds application in lots of the products we use in our everyday life.
Another area where separation techniques are useful is in the separating of blood into plasma and red cells. Also,
Separation chemistry plays an important role when alpha-and beta-emitting natural and artificial radionuclides at low levels must be determined in complex environmental matrices.
source

tl;dr:
| Separation technique | |
|---|---|
| Evaporation | Soluble solid (that does not decompose on heating) from liquid |
| Crystallization | Soluble solid (that decomposes on heating) from liquid |
| Filtration | An insoluble solid from liquid |
| Sieving | Solids of different sizes |
| Simple distillation | Miscible liquids whose boiling point different is much |
| Fractional distillation | Miscible liquids with close boiling points |
| Separating funnel | Immiscible liquids |
| Sublimation | Solids that sublime from those that don't. |
Reference
Separation techniques
Separation Process on Wikipedia
Methods of separating mixtures
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on steemit chat or discord here. If you are from Nigeria, you may want to include the #stemng tag in your post. You can visit this blog by @stemng for more details.