We have been using soaps or detergents to clean the dirt out of our cloths. But have you ever wondered how does a soap cleans the dirt? What causes the cleansing action of soaps? Before knowing that we should have some idea about what is soap and what are its constituents. Let’s get dive into it.
Soap is sodium or potassium salt of a higher fatty acid and may be represented as RCOO- Na+, where R is an alkyl group having higher number of carbon atoms; normally >12. Examples include sodium stearate CH3(CH2)16COO-Na+, which is a major component of many bar soaps. When it is allowed to dissolved in water, it dissociates into RCOO- ions.However, it is still consists of two parts - a long hydrocarbon chain ‘R’ (also called non polar ‘tail’) which is hydrophobic i.e water repelling and a polar group COO-(also called polar-ionic ‘head’ which is hydrophillic i.e water loving.
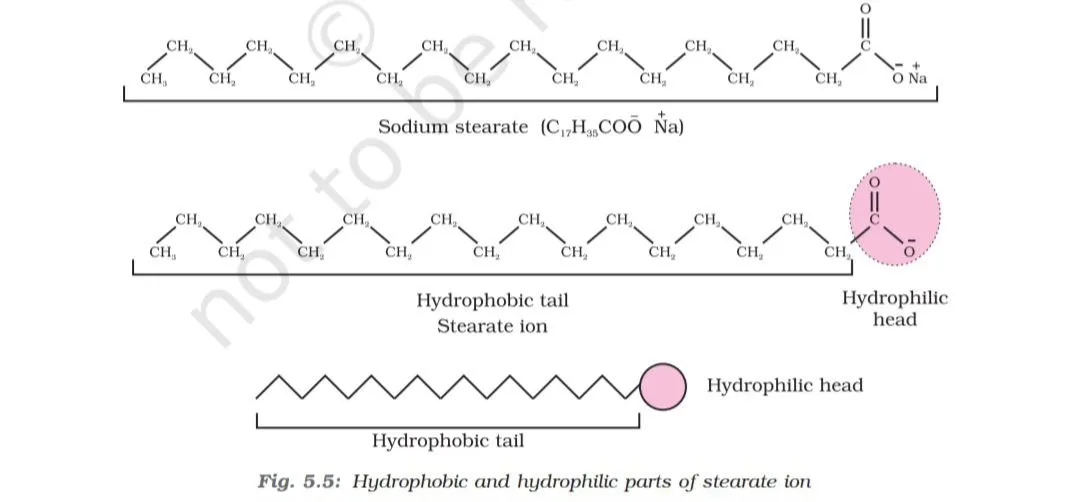
Source: NCERT
Now let’s have some idea regarding an another topic which is a must to understand the mechanism of action of soap. A colloid is a heterogenous system in which one substance is dispersed as very fine particles in another substance called dispersion medium. There are some substances which at low concentrations behave as normal strong electrolytes, but at higher concentrations exhibit colloidal behaviour due to the formation of aggregates. The aggregated particles thus formed are called micelles. The formation of micelles takes place only above a particular concentration called ‘critical micelle concentrations’ (CMC). On dilution these colloids revert back to individual ions. It is interesting to note that for soaps CMC is 10-4 to 10-3 mol L-1
The RCOO- ions are, therefore; present on the surface with their COO- groups in water and the hydrocarbon chains R staying away from it and remain at the surface. But at ‘CMC’, the anions are pulled into the bulk of solution and aggregates to form a spherical shape with their hydrocarbon chains pointing towards the centre of sphere with COO- part remaining outward on the surface of the sphere. An aggregate thus formed is known as ‘ionic micelle’. These micelle may contain as many as 100 such ions.
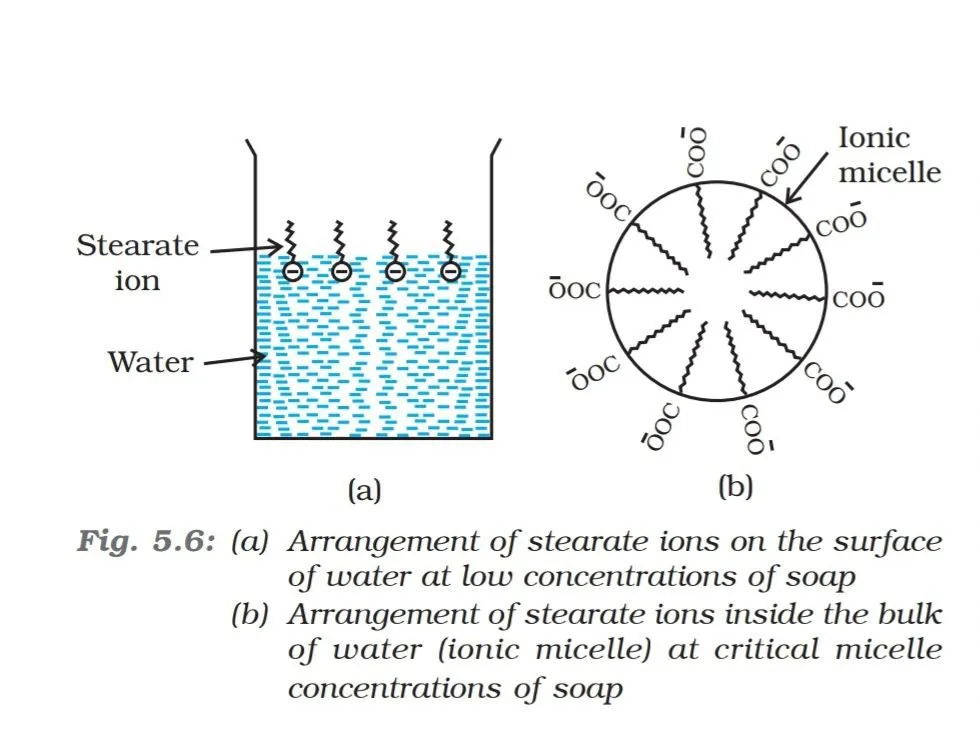
Source: NCERT
It has been mentioned earlier that a micelle consists of a hydrophobic hydrocarbon like central core. The cleansing action of soap is due to the fact that soap molecules form micelle around the oil droplet in such a way that hydrophobic part of the stearate ions is in the oil droplet and hydrophilic part projects out of the grease droplet like bristles. Since the polar groups can interact with water, the oil droplet surrounded by stearate ions is now pulled in water and removed from the dirty surface. Thus soap helps in emulsification and washing away of oils and fats. The negatively charged sheath around the globules prevents them from coming together and forming aggregates.
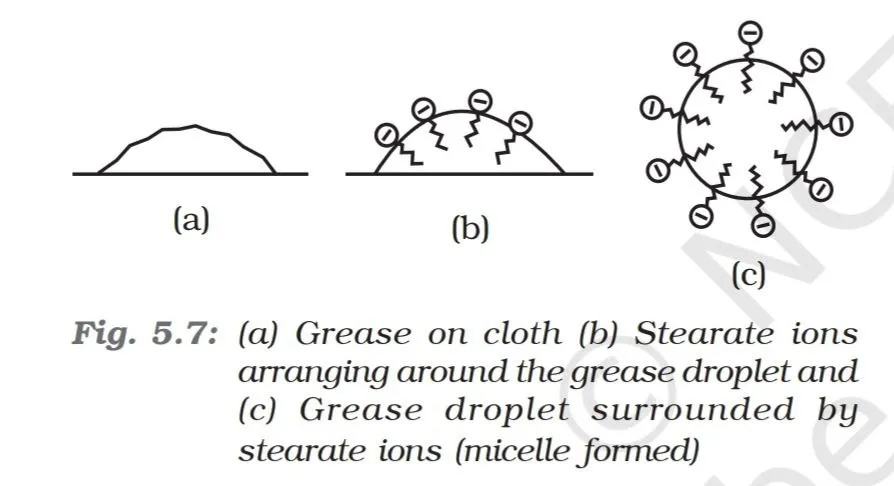
Source: NCERT